When salad oil is mixed with water and shaken, two layers quickly separate, the oily layer on
Question:
When salad oil is mixed with water and shaken, two layers quickly separate, the oily layer on top and the water layer on the bottom.
When an egg yolk (which is rich in lecithin, a phospholipid) is added and the mixture shaken, an emulsion is formed.
This means that the oil is suspended (not dissolved) in water as tiny particles that no longer form a separate layer.
Explain the action of the lecithin. (Addition of romaine lettuce, garlic, croutons, and Parmesan cheese yields a Caesar salad!)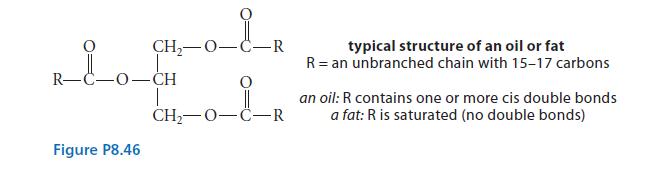
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





