Three hundred L/h of a 20 mole% C 3 H 8 80% n-C 4 H 10 gas
Question:
Three hundred L/h of a 20 mole% C3H8–80% n-C4H10 gas mixture at 0°C and 1.1 atm and 200 L/h of a 40 mole% C3H8–60% n-C4H10 mixture at 25°C and 1.1 atm are mixed and heated to 227°C at constant pressure. Enthalpies of propane and n-butane are listed below. Assume ideal-gas behavior.
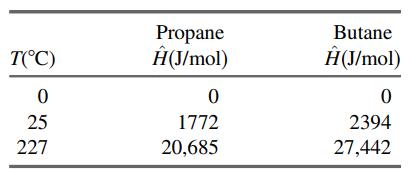
(a) Calculate the heat requirement in kJ/h.
(b) Where in your calculations did you use the ideal-gas assumption?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Elementary Principles of Chemical Processes
ISBN: 978-1119498759
4th edition
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Question Posted:





