Use the following vapor pressures of propane given here to calculate the enthalpy of vaporization using a
Question:
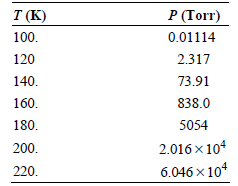
Transcribed Image Text:
P (Torr) T (K) 0.01114 100. 120 2.317 140. 73.91 160. 838.0 5054 180. 2.016 x 104 200. 6.046 x 10* 220.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (15 reviews)
A least squares fit of ln P ...View the full answer

Answered By

Pranav Makode
I am a bachelor students studying at professor ram meghe institute of technology and research. I have a great experience of being an expert. I have worked as an expert at helloexperts and solvelancer as a part time job. I have also worked as a doubt solver at ICAD SCHOOL OF LEARNING, which is in Amravati city. I have also worked as an Freelancer.
I have great experience of helping students, as described above. I can help any students in a most simple and understandable way. I will not give you have any chance for complaint. You will be greatfull to accept me as an expert.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A liquid mixture containing 25 mol% benzene and 75 mol% ethyl alcohol, in which components are miscible in all proportions, is heated at a constant pressure of 1 atm (101.3 kPa, 760 tort) from a...
-
Repeat Example 4.15 for temperatures corresponding to the following vapor pressures for solid PA: (a) 0.7 torr (b) 0.4 torr (c) 0.1 torr Plot the percent recovery of PA versus the solid vapor...
-
Your task in this problem will be to use a spreadsheet to generate a Txy diagram for a two-component system, using Raoult?s law to express the vapor?liquid equilibrium distribution of each species....
-
Ancient Indians did not believe in war and violence when it came it an expansion of political territory but relied on peaceful negotiation and strategy. Please explain your views on this quote with...
-
1. Using ERG theory, explain the reasons for the situation described in the case (The Motivation of a Rhodes Scholar). - Existence needs (E): - Relatedness needs (R): - Growth needs (G): 2. Using...
-
What are the main components of a debt contract? What alternative structures exist?
-
Following are errors, frauds, or other circumstances that an auditor might encounter as a result of applying audit tests to capital stock as of December 31, the balance sheet date: a. Shareholders'...
-
Capital Project Transactions. In 2011, Falts City began work to improve certain streets to be financed by a bond issue and supplemented by a federal grant. Estimated total cost of the project was...
-
Describe how expectancy and equity theories apply to compensation. Give an example of the types of reinforcement used. Compare the external and internal job evaluation methods.
-
Analyzing the Effects of Transactions Using T-Accounts, Preparing a Balance Sheet, and Evaluating the Current Ratio over Time as a Bank Loan Officer Strauderman Delivery Company, Inc., was organized...
-
The vapor pressure of a liquid can be written in the empirical form known as the Antoine equation, where A(1), A(2), and A(3) are constants determined from measurements: Starting with this equation,...
-
The vapor pressure of liquid benzene is 20,170 Pa at 298.15 K, and Î H vaporization =30.72 kJ mol -1 at 1 atm pressure. Calculate the normal and standard boiling points. Does your result for...
-
The positive integral powers of a square matrix A are defined as follows: Suppose that r and s are positive integers. Prove that A r A s = A r+s and that (A r ) s = A rs (in close analogy with the...
-
Risk management 1. Explain and support the kind of shutdown you would implement in this circumstance. In two sentences 2. Describe the risks the lockdown addresses and how it affects the frequency or...
-
Discuss how economic growth in India (or other emerging economies) affect United States and other emerging economies such as China by considering the effects of China's economic growth on United...
-
Suppose your gun has a muzzle speed of 10 m/s. You fire it horizontally off of a 25 m cliff. How far will the bullet go horizontally before hitting the ground? How long will it take? 2. You fire a...
-
What state based variables will change and which will not change when light waves trasition from air to plastic?
-
Could exchange rate fluctuations affect the regression models offered by Dr. Dubin in the Bali case? Hint: if exchange rate fluctuations were not included in the regression models by Dubin (or by...
-
Use (a) The Midpoint Rule and (b) Simpsons Rule to approximate the given integral with the specified value of n. (Round your answers to six decimal places.) Compare your results to the actual value...
-
Match the following. Answers may be used more than once: Measurement Method A. Amortized cost B. Equity method C. Acquisition method and consolidation D. Fair value method Reporting Method 1. Less...
-
Find the Laplace transform of cos 2 (at).
-
Find theLaplace transform of the function f(t) = e at where a is a constant.
-
Show that L{t cos (kt)} = (s 2 k 2 )/(s 2 + k 2 ) 2 .
-
1. Identifield and explain 3 causes of stress and 3 techniques to overcome stress ? 2. examine worksite stress management programmers more likely to be effective.
-
Bright Books is a bookstore chain established 10 years ago in northern Michigan. Bright Books has had great success in Michigan and Ohio and is beginning to expand into other states. Most recently,...
-
Design a synchronous counter using D flip-flops such that the count sequence of the counter follows the digits in . Each state must be 4-bit. Follow the steps below: (i) (ii) (iii) (iv) 137836...

Study smarter with the SolutionInn App


