Use the vapor pressures of n-butane given in the following table to calculate the enthalpy of vaporization
Question:
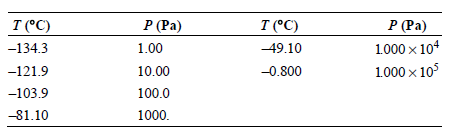
Transcribed Image Text:
P (Pa) 1000 x 104 1000 x 105 T (°C) -134.3 P (Pa) T (°C) 49.10 1.00 -0.800 -121.9 10.00 -103.9 100.0 -81.10 1000.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 73% (15 reviews)
A least squares fit of ...View the full answer

Answered By

Shubhradeep Maity
I am an experienced and talented freelance writer passionate about creating high-quality content. I have over five years of experience working in the field and have collaborated with several renowned companies and clients in the SaaS industry.
At Herman LLC, an online collective of writers, I generated 1,000+ views on my content and created journal content for 100+ clients on finance topics. My efforts led to a 60% increase in customer engagement for finance clients through revamping website pages and email interaction.
Previously, at Gerhold, a data management platform using blockchain, I wrote and published over 50 articles on topics such as Business Finance, Scalability, and Financial Security. I managed four writing projects concurrently and increased the average salary per page from $4 to $7 in three months.
In my previous role at Bernier, I created content for 40+ clients within the finance industry, increasing sales by up to 40%.
I am an accomplished writer with a track record of delivering high-quality content on time and within budget. I am dedicated to helping my clients achieve their goals and providing exceptional results.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Use the vapor pressures of SO 2 (l) given in the following table to calculate the enthalpy of vaporization using a graphical method or a least squares fitting routine. T (K) 190. P (Pa) T (K) 230. P...
-
Use the vapor pressures of tetrachloromethane given in the following table to calculate the enthalpy of vaporization using a graphical method or a least squares fitting routine. P/Pa T (K) 320. 330....
-
Use the following vapor pressures of propane given here to calculate the enthalpy of vaporization using a graphical method or a least squares fitting routine. P (Torr) T (K) 0.01114 100. 120 2.317...
-
Amandeep, a graduate of Cambrian Colleges Business Program, found a job as an online marketing specialist for Tangerine Bank. Within a year, he had saved {A} and decided to buy a new car but was not...
-
Read the Case study (Proctor & Gamble) and answer the following: 1. In your own words, summarize what went RIGHT or WRONG in this case? 2. Describe the Project Management process and practices used...
-
It has been estimated that one in five Americans suffers from allergies. The president of Hargrove University plans to randomly select 10 students from the undergraduate population of 1400 to attend...
-
Consumer complaints are frequently reported to the Better Business Bureau (BBB). Industries with the most complaints to the BBB are often banks, cable and satellite television companies, collection...
-
Pierre Company has a 12% note payable with a carrying value of $20,000. Pierre applies the fair value option to this note; given an increase in market interest rates, the fair value of the note is...
-
Describe your efforts and the results of your own operant conditioning experiment. Any behaviors conditioned should be positive and not harmful to the subject of your experiment.
-
John and Margaret Read the case study and prepare a 1012 minute video including your analysis of John and Margarets goals and concerns, along with questions for them, and potential recommendations...
-
The variation of the vapor pressure of the liquid and solid forms of a pure substance near the triple point are given by ln P solid /Pa = -8750K/T + 34.143 and In P liquid /Pa = -4053K/T + 21.10....
-
At 298.15 K, G f (HCOOH, g) = -351.0 kJ mol -1 and G f (HCOOH, l) 361.4 kJ mol -1 . Calculate the vapor pressure of water at this temperature.
-
Explain which is more stable, cis-1, 2-dimethylcyclopropane or trans-1, 2-dimethylcyclopropane.
-
Identify the research scenario, including the general area of focus. Develop a hypothetical research scenario that would necessitate the use of the Qualitative Method and the Grounded Theory...
-
Briefly discuss any limitations associated with this research scenario and the specific design. Develop a hypothetical research scenario that would necessitate the use of a Pretest and Posttest...
-
Explain from a technical viewpoint why it is important to distinguish a method, research, approach, and design. Next, briefly discuss how understanding each term individually in addition to how these...
-
Discuss the major threats to validity associated with this design and type of research (experimental or quasi-experimental). How will these threats be addressed, based on the discussion of the...
-
What are the advantages to including a cutoff score as a means of assignment in the regression-discontinuity approach?
-
Explain vicarious liability and any limitation on its availability.
-
In Exercises 1558, find each product. (9 - 5x) 2
-
An object moves through a fluid in the x direction. The only force acting on the object is a frictional force that is proportional to the negative of the velocity: Write the equation of motion of the...
-
Repeat the calculation of the previous example with a = 0.500 s 1 . Show that a narrower line width occurs.
-
Find the one-sided Fourier sine transform of the function f(x) = x.
-
es Hart, Attorney at Law, experienced the following transactions in Year 1, the first year of operations: 1. Accepted $16,600 on April 1, Year 1, as a retainer for services to be performed evenly...
-
Dahlia Corporation has a current accounts receivable balance of $439,516. Credit sales for the year just ended were $5,503,810. a. What is the receivables turnover? Note: Do not round intermediate...
-
Why does the organizational structure hold political significance? Provide an in-depth analysis of this concept using examples from both academic literature and real-world instances. Additionally,...

Study smarter with the SolutionInn App


