Question: Class Management | Help EXPERT a Problem Status Homework #11 Begin Date: 11/10/2025 5:01:00 PM Due Date: 11/17/2025 11:59:00 PM End Date: 12/31/2025 11:59:00 PM
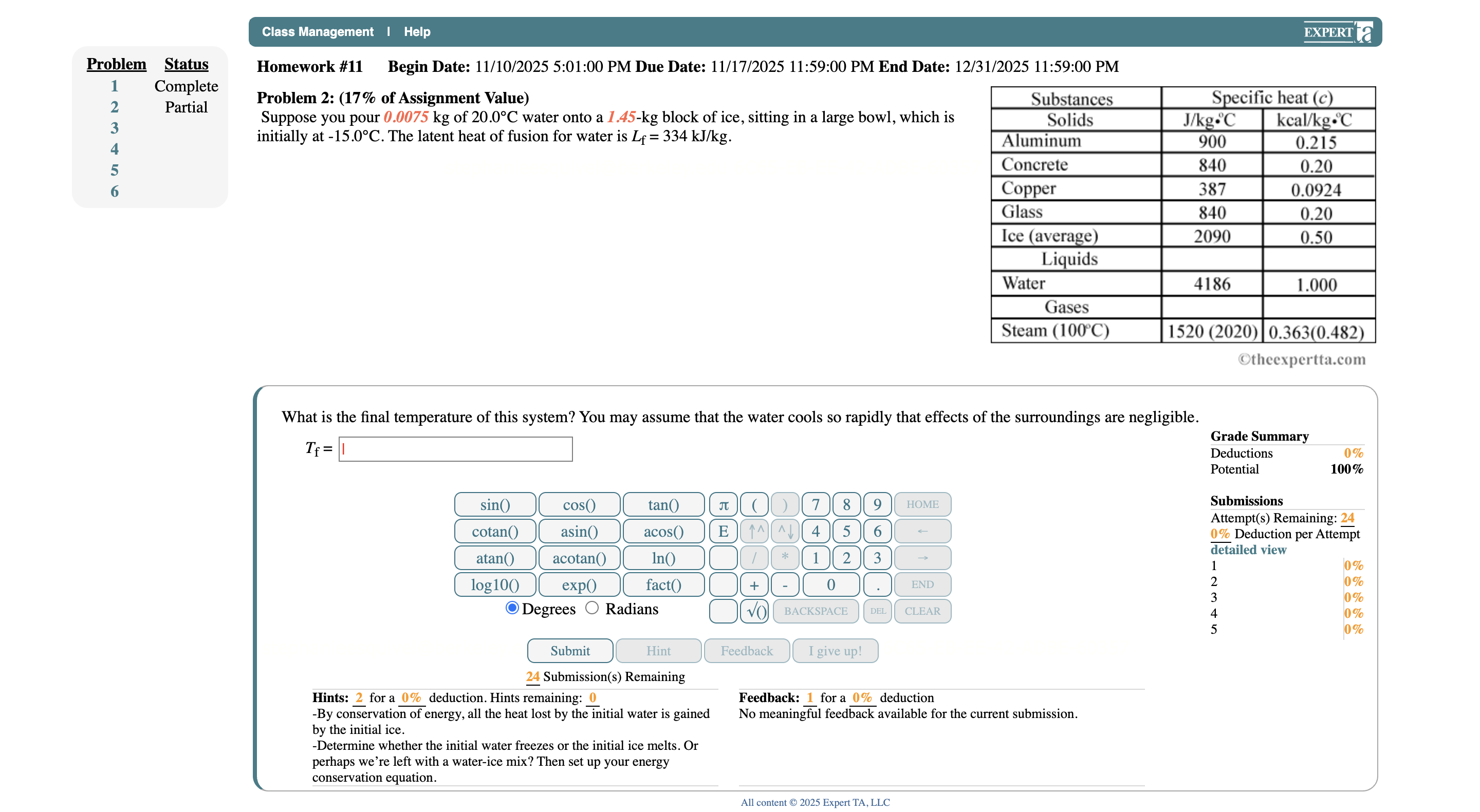 Class Management | Help EXPERT a Problem Status Homework #11 Begin Date: 11/10/2025 5:01:00 PM Due Date: 11/17/2025 11:59:00 PM End Date: 12/31/2025 11:59:00 PM Complete Partial Problem 2: (17% of Assignment Value) Substances Specific heat (c) Suppose you pour 0.0075 kg of 20.0 C water onto a 1.45-kg block of ice, sitting in a large bowl, which is Solids J/kg.C kcal/kg.C aUAWNH initially at -15.0 C. The latent heat of fusion for water is Lf = 334 kJ/kg. Aluminum 900 0.215 Concrete 840 0.20 Copper 387 0.0924 Glass 340 0.20 Ice (average) 2090 0.50 Liquids Water 4186 1.000 Gases Steam (100 C) 1520 (2020) 0.363(0.482) Otheexpertta.com What is the final temperature of this system? You may assume that the water cools so rapidly that effects of the surroundings are negligible. Grade Summary If= 1 Deductions 0% Potential 100% sin() cos() tan() 8 9 HOME Submissions Attempt(s) Remaining: 24 cotan( asin() acos() E 4 5 6 0% Deduction per Attempt detailed view atan acotan() In() * 1 2 3 0% log 10( exp() fact() 0 END 0% UAWNH 0% O Degrees O Radians BACKSPACE DEL CLEAR 0% 0% Submit Hint Feedback I give up! 24 Submission(s) Remaining Hints: 2 for a 0% deduction. Hints remaining: 0 Feedback: 1 for a 0% deduction By conservation of energy, all the heat lost by the initial water is gained No meaningful feedback available for the current submission. by the initial ice. -Determine whether the initial water freezes or the initial ice melts. Or perhaps we're left with a water-ice mix? Then set up your energy conservation equation. All content @ 2025 Expert TA, LLC
Class Management | Help EXPERT a Problem Status Homework #11 Begin Date: 11/10/2025 5:01:00 PM Due Date: 11/17/2025 11:59:00 PM End Date: 12/31/2025 11:59:00 PM Complete Partial Problem 2: (17% of Assignment Value) Substances Specific heat (c) Suppose you pour 0.0075 kg of 20.0 C water onto a 1.45-kg block of ice, sitting in a large bowl, which is Solids J/kg.C kcal/kg.C aUAWNH initially at -15.0 C. The latent heat of fusion for water is Lf = 334 kJ/kg. Aluminum 900 0.215 Concrete 840 0.20 Copper 387 0.0924 Glass 340 0.20 Ice (average) 2090 0.50 Liquids Water 4186 1.000 Gases Steam (100 C) 1520 (2020) 0.363(0.482) Otheexpertta.com What is the final temperature of this system? You may assume that the water cools so rapidly that effects of the surroundings are negligible. Grade Summary If= 1 Deductions 0% Potential 100% sin() cos() tan() 8 9 HOME Submissions Attempt(s) Remaining: 24 cotan( asin() acos() E 4 5 6 0% Deduction per Attempt detailed view atan acotan() In() * 1 2 3 0% log 10( exp() fact() 0 END 0% UAWNH 0% O Degrees O Radians BACKSPACE DEL CLEAR 0% 0% Submit Hint Feedback I give up! 24 Submission(s) Remaining Hints: 2 for a 0% deduction. Hints remaining: 0 Feedback: 1 for a 0% deduction By conservation of energy, all the heat lost by the initial water is gained No meaningful feedback available for the current submission. by the initial ice. -Determine whether the initial water freezes or the initial ice melts. Or perhaps we're left with a water-ice mix? Then set up your energy conservation equation. All content @ 2025 Expert TA, LLC
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


