Question: Consider a reaction that consumes reactant C. Below are two plots of the same data set for two different functions containing the concentration of
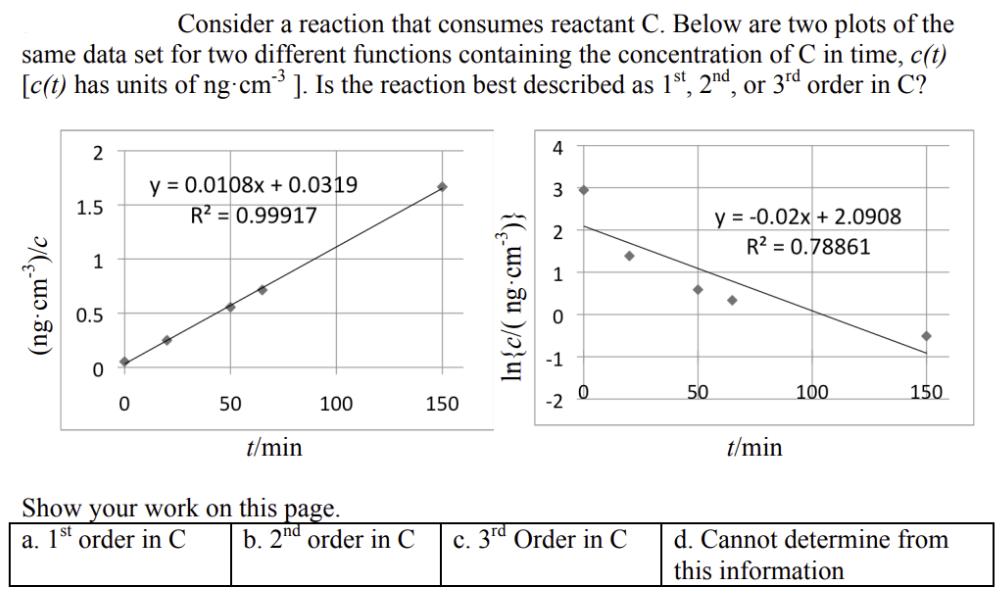
Consider a reaction that consumes reactant C. Below are two plots of the same data set for two different functions containing the concentration of C in time, c(t) [c(t) has units of ng cm ]. Is the reaction best described as 1st, 2nd, or 3rd order in C? (ng-cm)/c 2 1.5 1 0.5 0 y = 0.0108x + 0.0319 R = 0.99917 50 t/min 100 Show your work on this page. a. 1st order in C b. 2nd order in C 150 In{c/(ng-cm)} 4 3 -2 0 c. 3rd Order in C 50 y = -0.02x + 2.0908 R = 0.78861 t/min 100 150 d. Cannot determine from this information
Step by Step Solution
There are 3 Steps involved in it
To determine the order of the reaction with respect to reactant C w... View full answer

Get step-by-step solutions from verified subject matter experts


