Question: Problem 1 (10 points): Pumps are routinely required to increase pressure of liquids. In one process, 2.5 GPM of ethanol liquid must be pumped
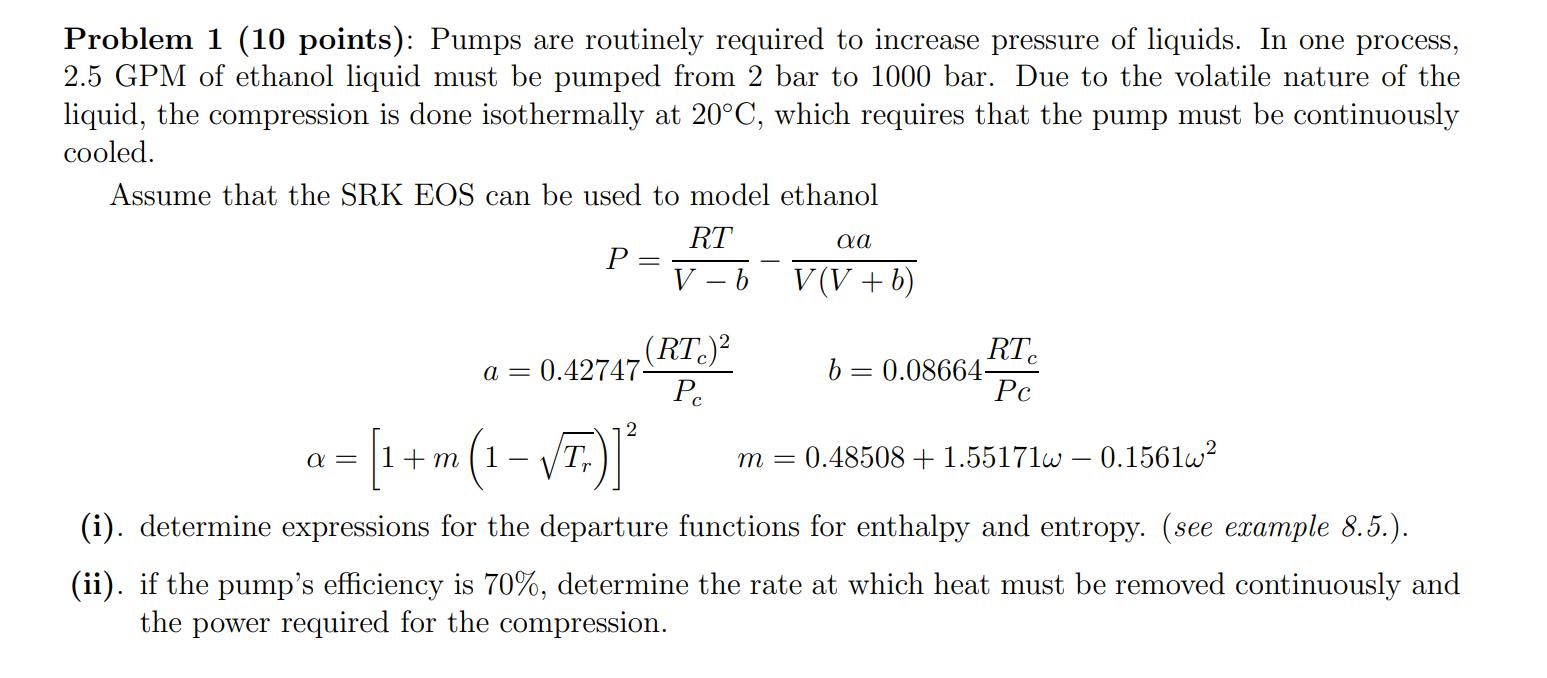
Problem 1 (10 points): Pumps are routinely required to increase pressure of liquids. In one process, 2.5 GPM of ethanol liquid must be pumped from 2 bar to 1000 bar. Due to the volatile nature of the liquid, the compression is done isothermally at 20C, which requires that the pump must be continuously cooled. Assume that the SRK EOS can be used to model ethanol RT P V b V(V+b) (RTC) RT a = 0.42747- b = 0.08664- Pc Pc 2 a-[1+m(1-T)]* m = 0.48508 +1.55171w - 0.1561w (i). determine expressions for the departure functions for enthalpy and entropy. (see example 8.5.). (ii). if the pump's efficiency is 70%, determine the rate at which heat must be removed continuously and the power required for the compression.
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


