The measured molar heat capacities for crystalline KCl are as follows at the indicated temperatures: a. Explain
Question:
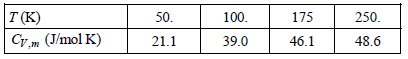
a. Explain why the high-temperature limit for CV is apparently twofold greater than that predicted by the Dulong€“Petit law.
b. Determine if the Einstein model is applicable to ionic solids. To do this uses the value for CV at 50. K to determine ΘV, then uses this temperature to determine CV at 175 K.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





