Question: A binary ideal-gas mixture of A and B undergoes an isothermal, isobaric separation at To, the infinite surroundings temperature. Starting with Eq. (4), Table 2.1,
A binary ideal-gas mixture of A and B undergoes an isothermal, isobaric separation at To, the infinite surroundings temperature. Starting with Eq. (4), Table 2.1, derive an equation for the minimum work of separation, Wmin,in terms of mole fractions of the feed and the two products. Use your equation to prepare a plot of the dimensionless group, Wmin,/RTonF, as a function of mole fraction of A in the feed for:(a) A perfect separation(b) A separation with SFA = 0.98, SFB = 0.02(c) A separation with SRA = 9.0 and SRB = 1/9(d) A separation with SF = 0.95 for A and SPA,B = 361How sensitive is Wmin to product purities? Does Wmin, depend on the particular separation operation used? Prove, by calculus, that the largest value of Wmin occurs for a feed with equimolar quantities of A andB.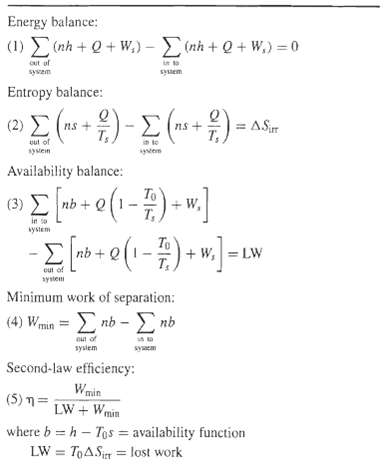
Energy balance: ) h +0+ W)- m+0+ .)-0 cut uf in sysem syaem Entropy balance: "9)-)- = ASir (2) +s ns uut of in to yten Availability balance: To nb + Q in so system -(- ) To + W, LW eut of sytem Minimum work of separation: (4) Wmin = E nb - nb on of syslem syaem Second-law efficiency: Wmin LW + Wmin (5) n= where b = h - Tos = availability function LW = TOASir = lost work
Step by Step Solution
3.27 Rating (171 Votes )
There are 3 Steps involved in it
Combining Eqs 1 and 2 for one feed F in and two products P 1 and P 2 out However for isothermal sepa... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
37-E-C-E-S-P (34).docx
120 KBs Word File


