A charge of 100 lbmol of benzene (B), monochlorobenzene (MCB), and o-dichlorobenzene (DCB) is being distilled in
Question:
A charge of 100 lbmol of benzene (B), monochlorobenzene (MCB), and o-dichlorobenzene (DCB) is being distilled in a batch rectifier that consists of a total condenser, a column with 10 theoretical stages, and a partial reboiler. Following the establishment of total reflux, the first operation step begins for a boilup rate of 200 lbmol/h and a reflux ratio of about 3. At the end of 0.60 h, the following conditions exist for the top three stages in the column:
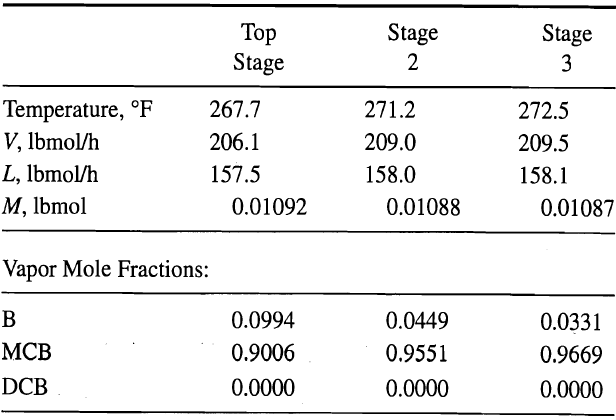
Liquid Mole Fractions:
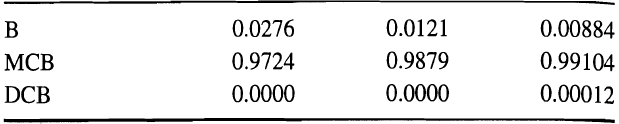
In addition reboiler and condenser holdups at 0.6 h are 66.4 and 0.1113 lbmol, respectively. For benzene, use the preceding data with (13-36) and (13-39) to estimate the liquid-phase mole fraction of benzene leaving Stage 2 at 0.61 h by using the explicit-Euler method with a ∆t of 0.01 h. If the result is unreasonable, explain why with respect to stability and stiffness considerations.
Step by Step Answer:






