Question: A cylindrical liquid oxygen (LOX) tank has a diameter of 4 ft, a length of 20 ft, and hemispherical ends. The boiling point of LOX
A cylindrical liquid oxygen (LOX) tank has a diameter of 4 ft, a length of 20 ft, and hemispherical ends. The boiling point of LOX is ?? 297?F. An insulation is sought which will reduce the boil-off rate in the steady state to no more than 25 lb/h. The heat of vaporization of LOX is 92 Btu/lb. If the thickness of this insulation is to be no more than 3 in., what would the value of it??s thermal conductivity have to be?GIVENInsulated cylindrical tank with hemispherical ends filled with LOXDiameter of tank (Dt) = 4 ftLength of tank (Lt) = 20 ftBoiling point of LOX (Tbp) = ??297?FHeat of vaporization of LOX (hfg) = 92 Btu/lbSteady state boil-off rate ( m) = 25 lb/hMaximum thickness of insulation (L) = 3 in. = 0.25 ftASSUMPTIONSThe length given includes the hemispherical endsThe thermal resistance of the tank is negligible compared to the insulationThe thermal resistance at the interior surface of the tank isnegligible
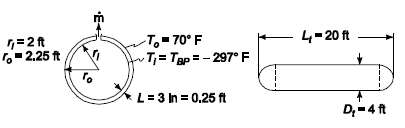
4-20 ft T.-70 F T= Tap = - 297 F To ,=2 t 2.25 ft L= 3 In = 0.25 ft D;-4 ft
Step by Step Solution
3.35 Rating (167 Votes )
There are 3 Steps involved in it
The tank can be thought of as a sphere the ends separa... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
66-E-M-E-H-M-T (1489).docx
120 KBs Word File


