Question: (a) If the nucleus in Example 19-4 had a charge of +2e (as would be the case for a nucleus of helium),would the speed of
(a) If the nucleus in Example 19-4 had a charge of +2e (as would be the case for a nucleus of helium),would the speed of the electron be greater than, less than, or the same as that found in the Example? Explain. (Assume the radius of the electron's orbit is the same.)
(b) Find the speed of the electron for a nucleus of charge +2e.
Example 19-4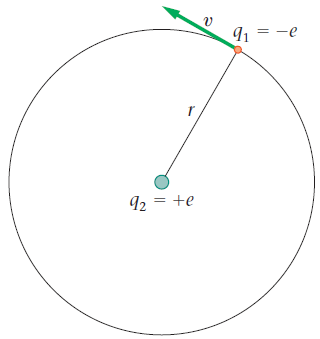
91 = -e 92 = +e
Step by Step Solution
3.47 Rating (167 Votes )
There are 3 Steps involved in it
Picture the Problem An electron orbits with speed v at a distance r ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
1307-P-M-P-Q-M(530).docx
120 KBs Word File


