Question: A liquid is placed in a well-insulated container, which is then sealed. Initially, the container and its contents (the Liquid and pure nitrogen) are at
A liquid is placed in a well-insulated container, which is then sealed. Initially, the container and its contents (the Liquid and pure nitrogen) are at 93?C and 1 atm: the liquid volume is 70cm3, and the gas volume is 3.00 L. The liquid partially evaporates, and the system cools down and eventually comes to thermal equilibrium at 85?C with Liquid still present. Physical property data for the liquid and its vapor are:
(a) Determine (Cv)liq and (Cv)vap (See Equations 8.3-11 and 8.3-12.)
(b) Draw and label a flowchart for this closed system process, and write and simplify the energy balance equation, assuming adiabatic operation.
(c) Use the energy balance to calculate the mass of liquid that evaporates, taking 4.97 cal/(mol??C) as the heat capacity of nitrogen.
(d) Calculate the vapor pressure of the Liquid at 85?C, assuming that the gas volume remains Constant at 3.00 L.
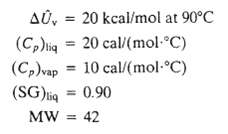
AU, (Cp)liq = 20 cal/(mol-C) 10 cal/(mol-C) (Cp)vap (SG)liq = 0.90 MW = 42 20 kcal/mol at 90C
Step by Step Solution
3.33 Rating (171 Votes )
There are 3 Steps involved in it
a b Cp Cp 20 calmol C C C R102 no no mol N 300 L 93C 1 atm 7 mol A1 070 m... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
13-E-C-E-C-P (469).docx
120 KBs Word File


