A methanolwater feed stream is introduced to a vaporizer in which a molar fraction f of the
Question:
A methanol–water feed stream is introduced to a vaporizer in which a molar fraction f of the feed is vaporized. The feed has a methanol mole fraction of xF = 0.4, and the vaporizer operates at a pressure of 1 atm absolute and 80°C. Vapor and liquid leaving the device are in equilibrium at the temperature and pressure of the system and have methanol mole fractions of y and x, respectively.
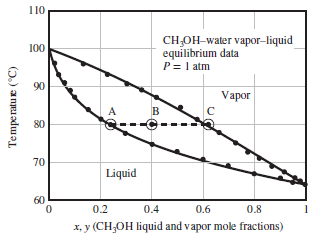
A Txy diagram for methanol–water mixtures at 1 atm absolute is shown below. The feed to the vaporizer and the liquid and vapor product streams are shown as points B, A, and C, respectively.
(a) Prove that f can be determined from the equation
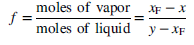
Use this result to determine f for the specific conditions cited above (xF = 0:4; T = 80°C).
(b) Use the Txy diagram to estimate the minimum and maximum temperatures at which the given feed stream could be separated into vapor and liquid fractions at 1 atm. In each case, what fraction of the feed would be vaporized?
(c) The vapor at C is sent to a condenser operated at constant pressure (1 atm). The liquid and vapor product streams leaving the condenser are in equilibrium and in a ratio of 1 mol vapor/1 mol liquid. Estimate the temperature and compositions of the two streams leaving the condenser.
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-1119498759
4th edition
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard





