A mixture of n-heptane (H) and toluene (T) is separated by extractive distillation with phenol (P). Distillation
Question:
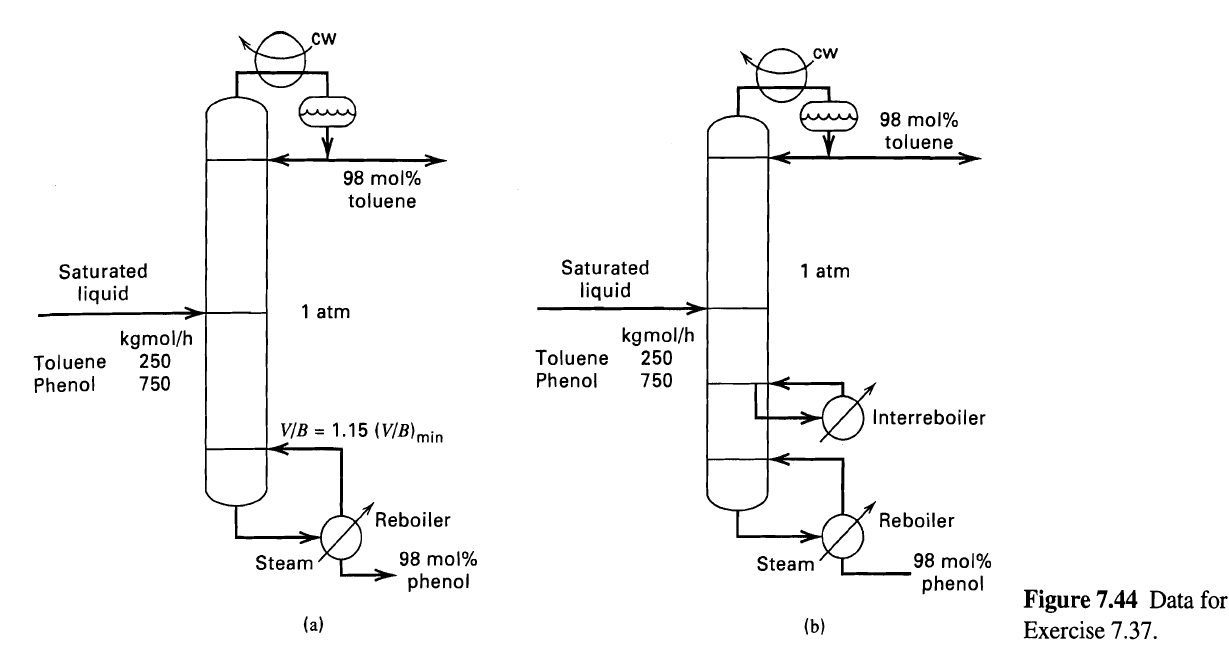
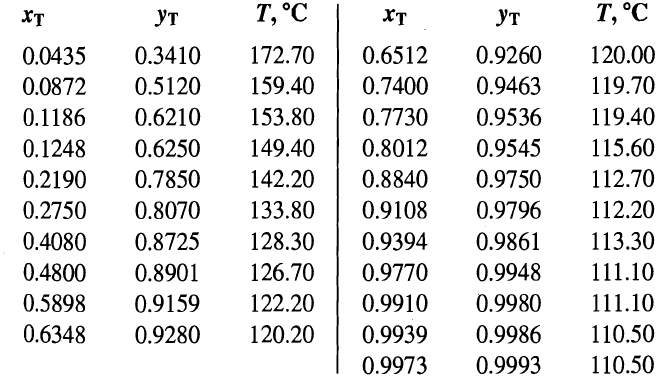
A mixture of n-heptane (H) and toluene (T) is separated by extractive distillation with phenol (P). Distillation is then used to recover the phenol for recycle as shown in Figure 7.44a, where the small amount of n-heptane in the feed is ignored. For the conditions shown in Figure 7.44a, determine the number of theoretical stages required. Note that heat will have to be supplied to the reboiler at a high temperature because of the high boiling point of phenol. Therefore, consider the alternative scheme in Figure b, where an interreboiler, located midway between the bottom plate and the feed stage, is used to provide 50% of the boilup used in Figure a. The remainder of the boilup is provided by the reboiler. Determine the number of theoretical stages required for the case with the interreboiler and the temperature of the interreboiler stage. Unsmoothed vapor-liquid equilibrium data at 1 atm are
Step by Step Answer:






