Question: (a) Show that the total binding energy Ebe of a given nuclide is Ebe = ZH + Nn - , where H is the mass
(a) Show that the total binding energy Ebe of a given nuclide is Ebe = ZΔH + NΔn - Δ, where ΔH is the mass excess of 1H, Δn, is the mass excess of a neutron, and A is the mass excess of the given nuclide.
(b) Using this method, calculate the binding energy per nucleon for 197Au. Compare your result with the value listed in Table. The needed mass excesses, rounded to three significant figures, are Δ H = + 7.29 MeV, Δ n = +8.07 MeV and Δ197 = -31.2MeV.
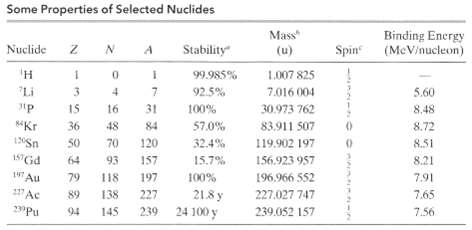
Some Properties of Selected Nuclides Mass Binding Energy (MeV/nucleon) Spin Nuclide Stability (u) 'H 99.985% 1.007 825 "Li 92.5% 7.016 004 4 5.60 IS 16 31 100% 30.973 762 8.48 Kr 36 57.0% 83.911 507 8.72 48 84 50 70 120 32.4% 119.902 197 8.51 ASGd 157 64 93 15.7% 156.923 957 8.21 147 Au 196,966 552 79 118 197 100% 7.91 227Ac 227,027 747 138 227 21.8 y 7.65 89 24 100 y 7.56 94 145 239 239.052 157
Step by Step Solution
3.25 Rating (163 Votes )
There are 3 Steps involved in it
If a nucleus contains Z protons and N neutrons its bindin... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
2-P-M-P-N-P (200).docx
120 KBs Word File


