Question: All the following reactions have been described in the chemical literature and proceed in good yield. In some cases the reactants are more complicated than
All the following reactions have been described in the chemical literature and proceed in good yield. In some cases the reactants are more complicated than those we have so far encountered. Nevertheless, on the basis of what you have already learned, you should be able to predict the principal product in each case.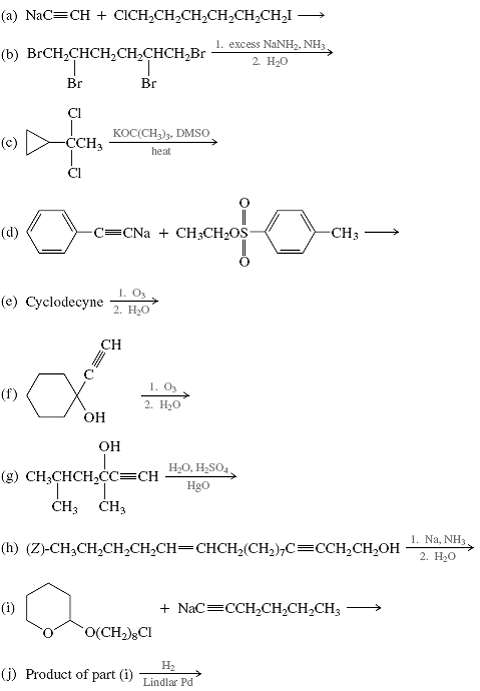
(a) NaC=CH + CCH,CH,CH,CH,CH CHI (b) BrCHCHCHCHCHCHBr (d) Br Cl CCH3 (e) Cyclodecyne (1) KOC(CH). DMSO heat -C=CNa + CH3CHOS Br 1. Os 2. HO CH - OH OH (g) CHCHCHCC=CH T CH3 CH3 1. Oy 2. HO (j) Product of part (i) O(CH), CI I. excess NaNH, NH3, 2 HO (h) (Z)-CHCHCHCHCH=CHCH(CH),C=CCHCHOH HO, HSO Hgo -CH- - H Lindlar Pd + NaC=CCHCHCHCH3 1. Na, NH3 2. HO
Step by Step Solution
3.30 Rating (153 Votes )
There are 3 Steps involved in it
a The dihaloalkane contains both a primary alkyl chloride and a primary alkyl iodide functional group Iodide is a better leaving group than chloride a... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
C-OC-A (72).docx
120 KBs Word File


