Ammonia, present at a partial pressure of 12 torr in an air stream saturated with water vapor
Question:
Ammonia, present at a partial pressure of 12 torr in an air stream saturated with water vapor at 68°F and 1 atm, must be removed to the extent of 99.6% by water absorption at the same temperature and pressure. Two thousand pounds of dry air per hour are to be handled.
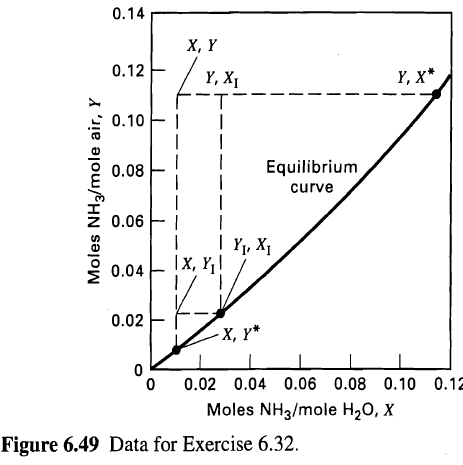
(a) Calculate the minimum amount of water necessary using the equilibrium data for Exercise 6.32 in Figure 6.49.
(b) Assuming an operation at 2 times the minimum water flow and at one-half the flooding gas velocity, compute the dimensions of a column packed with 38-mnl ceramic Berl Saddles.
(c) Repeat part (b) for 50-mm Pall rings.
(d) Which of the two packings would yourecommend?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





