Question: An aqueous slum? at 30?C containing 20.0 wt% solids is fed to an evaporator in which enough water is vaporized at 1 atm to produce
An aqueous slum? at 30?C containing 20.0 wt% solids is fed to an evaporator in which enough water is vaporized at 1 atm to produce a product slurry containing 35.0 wt% solids. Heat is supplied to the evaporator by feeding saturated steam at 1.6bar absolute into a coil immersed in the liquid. The steam condenses in the coil, and the slurry boils at the normal boiling point of pure water. The heat capacity of the solids may be taken to be half that of liquid water.
(a) Calculate the required steam feed rate (kg/h) for a slurry feed rate of 1.00 X 10 kg/h.
(b) Vapor re-compression is often used in the operation of an evaporator. Suppose that the vapor (steam) generated in the evaporator described above is compressed to 1.6 bar and simultaneously heated to the saturation temperature at 1.6bar, so that no condensation occurs. The compressed steam and additional saturated steam at 1.6bar are then fed to the evaporator coil, in which isobaric condensation occurs. How much additional steam is required?
(c) What more would you need to know to determine whether or not vapor re-compression is economically advantageous in this process?
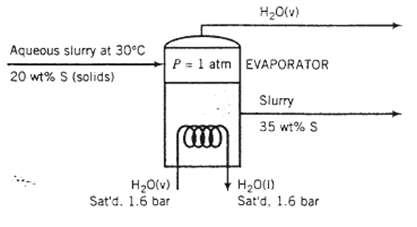
Aqueous slurry at 30C 20 wt% S (solids) HO(v) Sat'd. 1.6 bar HO(v) P= 1 atm EVAPORATOR (CIO) Slurry 35 wt% S HO(1) Sat'd, 1.6 bar
Step by Step Solution
3.41 Rating (164 Votes )
There are 3 Steps involved in it
a 1000 kgh 30C 0200 kg solidskg 0800 kg HO1kg mi kg HOvh 16 ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
13-E-C-E-C-P (466).docx
120 KBs Word File


