Question: Below are listed the atomic weight, density, and atomic radius for three hypothetical alloys. For each determine whether its crystal structure is FCC, BCC, or
Below are listed the atomic weight, density, and atomic radius for three hypothetical alloys. For each determine whether its crystal structure is FCC, BCC, or simple cubic and then justify your determination. A simple cubic unit cell is shown in Figure 3.24.
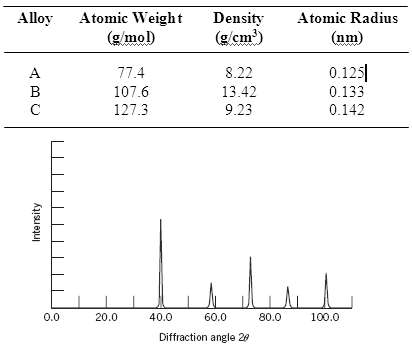
Alloy Atomic Weight Density (g/cm') Atomic Radius (g/mol) (nm) 0.125| 8.22 77.4 107.6 13.42 0.133 127.3 9.23 0.142 0.0 20.0 40.0 60.0 80.0 100.0 Diffraction angle 20 Intersity
Step by Step Solution
3.36 Rating (168 Votes )
There are 3 Steps involved in it
For each of these three alloys we need by trial and error to ca... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
33-E-M-S-E-M-S (37).docx
120 KBs Word File


