Question: Bond strengths can be used to estimate the relative stability of isomers that have different bonds. The isomer that has the larger total bond energy
Bond strengths can be used to estimate the relative stability of isomers that have different bonds. The isomer that has the larger total bond energy is more stable. One of the following isomers is more stable than the other. The less stable one is rapidly converted Co the more stable one, so it cannot be isolated. On the basis of bond dissociation energies, which of these two isomers is mores table?
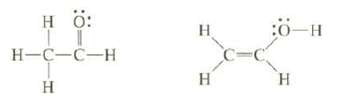
H : H-C-C-H H :- H
Step by Step Solution
3.32 Rating (155 Votes )
There are 3 Steps involved in it
The total bond energy of the product 669k calmol is ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
15-C-O-O-C (38).docx
120 KBs Word File


