Calculate the reduced mass for the H35Cl and H37Cl molecules and the fractional difference ??/. Show that
Question:
Calculate the reduced mass for the H35Cl and H37Cl molecules and the fractional difference ??μ/μ. Show that the mixture of isotopes in HCl leads to a fractional difference in the frequency of a transition from one rotational state to another given by ??f/f = ??μ/μ. Compute ??f/f and compare your result withFigure.
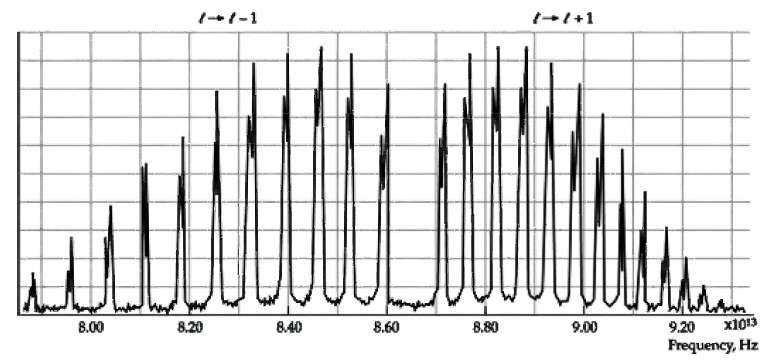
Transcribed Image Text:
1+1-1 x1013 8.20 8.40 B.00 8.60 8.80 9.00 9.20 Frequency, Hz
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 54% (11 reviews)
We shall express the results in unified mass units For ...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Fundamentals of Ethics for Scientists and Engineers
ISBN: 978-0195134889
1st Edition
Authors: Edmund G. Seebauer, Robert L. Barry
Question Posted:
Students also viewed these Modern Physics questions
-
Show that a mixture of saturated liquid water and saturated water vapor at 100C satisfies the criterion for phase equilibrium.
-
Show that a mixture of saturated liquid water and saturated water vapor at 300 kPa satisfies the criterion for phase equilibrium.
-
Show that a mixture of saturated liquid water and saturated water vapor at 300 kPa satisfies the criterion for phase equilibrium.
-
Various streptococci and lactobacilli were traditionally grouped together as lactic acid bacteria because of their characteristic fermentation. Most of them were found to have a DNA Guanine plus...
-
Record the following transactions at (a) gross and (b) net: 201X Sept. 3 Bought merchandise on account from Curik Co.; terms 3/10, n/30, $7,500. Voucher no. 32 was prepared. 18 Issued check no. 479...
-
A liquid refrigerant (sg = 1.08) is flowing at a weight flow rate of 28.5 N/h. Calculate the volume flow rate and the mass flow rate.
-
The following covenants are extracted from the indenture of a bond issue. The indenture provides that failure to comply with its terms in any respect automatically advances the due date of the loan...
-
As the comptroller of a hospital, you were just informed that one of the surgeons failed to remove an instrument from a patients innards. The hospital is certain to be sued. How should this...
-
Share a major issue or problem you had to overcome when working in a team or on a group project. What role (leader) did you play in the group or team? What steps did you take to improve the team...
-
Ace, Boy, and Cid are partners sharing profits in the ratio of 3:3:2. On July 31, their capital balances are as follows: Ace P700,000, Boy P500,000, and Cid P400,000. The partners agree to admit...
-
Two objects of mass m 1 and m 2 are attached to a spring of force constant K and equilibrium length r 0 . (a) Show that when m 1 is moved a distance ?r 1 from the center of mass, the force exerted by...
-
In calculating the rotational energy levels of a diatomic molecule, we did not consider rotation of the molecule about the line joining the atoms. (a) Estimate the moment of inertia of the H2...
-
The bookkeeper for Stan Tucci Equipment Repair made a number of errors in journalizing and posting, as described below. 1. A credit posting of $400 to Accounts Receivable was omitted. 2. A debit...
-
Suppose you apply the same amount of force (say, net force is 6 newtons) to two separate carts, one cart with a mass of 1 kg and the other with a mass of 2 kg. Which cart will accelerate more (go...
-
The International Space Station is in orbit around Earth at a distance r from the center of the Earth. A chunk of space debris hits the Earth by 12%. Did Earth's gravitational force on the Station...
-
An electron in the hydrogen atom makes a transition from the first energy level (E= -13.6eV) to the fifth energy level (E= -0.544 eV) by absorbing a photon. Calculate the frequency of the photon...
-
Supposed you are stopped for a traffic light, and an emergency response vehicle approaches you from behind. The siren produces a sound with a frequency of 955Hz. But the apparent (perceived)sound...
-
Find the value o f x . l o g 9 1 0 x + 5 = 1 8
-
Use the Lvy-Ciesielski representation \(B(t)=\sum_{n=0}^{\infty} G_{n} S_{n}(t), t \in[0,1]\), to obtain a series representation for \(X:=\int_{0}^{1} B(t) d t\) and find the distribution of \(X\).
-
Copy and complete the statement. 3800 m ? km =
-
(a) Find i s ,v 1 ,v 2 , and i l in Fig. 5-18(a). (b) Compare these results with those obtained when source and load are connected directly as in Fig. 5-18(b). R voltage source 11, R voltage source...
-
A certain electronic device needs to be protected against sudden surges in current. In particular, after the power is turned on the current should rise no more than 7.5mA in the first 120s. The...
-
A 25-turn 12.5 cm-diameter coil is placed between the pole pieces of an electromagnet. When the magnet is turned on, the flux through the coil changes, inducing and emf at what rate (in T/s) must the...
-
Calculate the peak output voltage of a simple generator whose square armature windings are 660 cm on a side the armature contains 155 loops and rotates in a field of 0.200T at a rate of 120 rev/s.
-
Select a participant (colleague, family member, or friend) that you would like to receive feedback from. Use the information from the table to plan a two-way, open, and evaluative feedback session as...
-
Imagine you are the assistant manager of the place in which you currently work. You have been instructed by the manager to organize and facilitate a company team building activity to promote...
-
Analyze key change management principles. The organization is having difficulties and frustration regarding outdated technology. Explain the purpose and discipline of change management. Outline a...

Study smarter with the SolutionInn App


