Question: Complete these equilibrium reaction sin the most reasonable manner possible using the curved arrow convention to show the movement of electrons in the reactions, Predict
Complete these equilibrium reaction sin the most reasonable manner possible using the curved arrow convention to show the movement of electrons in the reactions, Predict whether the reactants or the products are favored.
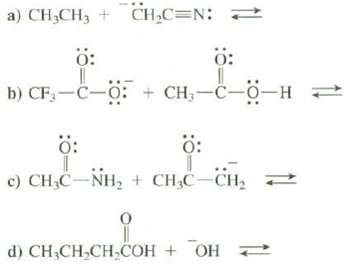
a) CH,CH, + CH,C=N: < + : D) CF-L-F b) CF-C-: : CH-C-0-H : : c) CHC-NH + CHC-CH 0 d) CHCHCHCOH + OH
Step by Step Solution
3.38 Rating (170 Votes )
There are 3 Steps involved in it
a CHCH is a weaker acid than CH3CN because the conjugate base ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
15-C-O-A-B-R (35).docx
120 KBs Word File


