Question: Compound A, C 6 H 12 O, has an IR absorption at 1715 cm ?1 and gives compound B, C6H15N, when treated with ammonia and
Compound A, C6H12O, has an IR absorption at 1715 cm?1 and gives compound B, C6H15N, when treated with ammonia and NaBH3CN. The IR and 1H NMR spectra of B are shown. What are the structures of A and B?
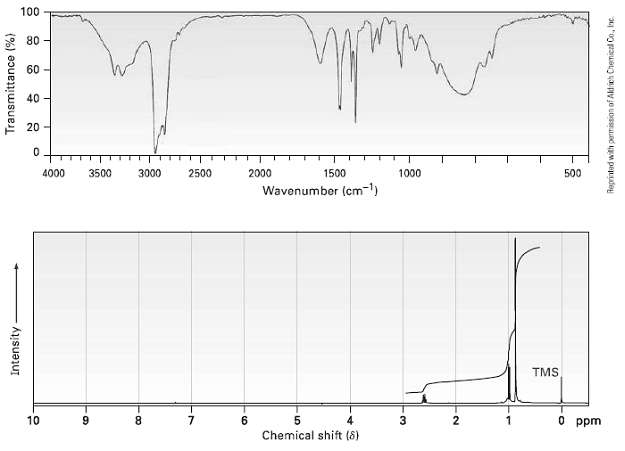
100 80 60 - 40 20 2000 4000 3500 3000 2500 1500 1000 500 Wavenumber (cm-) TMS O ppm 10 6. 6. Chemical shift (8) Transmittance (%) Intensity Reprinted with permission of Aldich Chemical Co., Inc
Step by Step Solution
3.45 Rating (181 Votes )
There are 3 Steps involved in it
The IR spectrum shows that B is a primary amin... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
22-C-O-A (74).docx
120 KBs Word File


