Cyclopropanone is highly reactive because of its large amount of angle strain, but methylcyclopropenone, although even more
Question:
Cyclopropanone is highly reactive because of its large amount of angle strain, but methylcyclopropenone, although even more strained than Cyclopropanone, is nevertheless quite stable and can even be distilled. Explain, taking the polarity of the carbonyl group intoaccount.
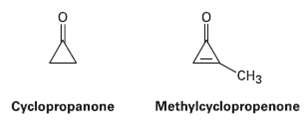
Transcribed Image Text:
"CHз Cyclopropanone Methylcyclopropenone
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (10 reviews)
00 A 8 0 CH3 B CH3 In resonance structure A methylcyclopropenone is a cyclic conjugated com...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
The polarization of a carbonyl group can be represented by a pair of resonance structures: Cyclopropenone and cycloheptatrienone are more stable than anticipated. Cyclopentadienone, however, is...
-
Explain why, for more than a decade, a massive amount of money flowed into the United States. Compare and contrast your explanation with that of the President. Most economists agree that the problems...
-
Because a center equilibrium is stable but not asymptotically stable, nonlinear perturbation can have different outcomes. shown in Problems I 1 and 12. 1. Determine the stability of the equilibrium...
-
Great ride (GR) is in the business of manufacturing and selling high-end vehicles. GR signs a deal with the CEO of a consulting firm for a luxury SUV. You are the long-time Controller for GreatRide...
-
What challenges does the system pose for drivers and their managers?
-
Develop an analysis that shows that increasing n for a rate 1/n code always degrades system performance in an AWGN. In order to obtain specific results, assume PSK modulation.
-
For each of the following situations, calculate a \(95 \%\) confidence interval for the mean ( \(\sigma\) known), beginning with the step, "Identify the critical value of \(z\)." X 50.00, X = 3.00 X...
-
Assume that Models and More store bought and sold a line of dolls during December as follows: Requirements 1. Compute the cost of cost of goods sold, cost of ending merchandise inventory, and gross...
-
TranscribedText: Problem 2. [25pts] For each of the following vector fields F find the curl. 1. (5pts) F : R3 -> R3 such that F I2 = I3 2TIT213 V X F(x) = 2. (5pts) F : R3 -> R3 such that F I2 = T2 +...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Draw an energy diagram for the three molecular orbitals of the cyclopropenyl system (C3H3). How are these three molecular orbitals occupied in the cyclopropenyl anion, cation, and radical? Which of...
-
Cycloheptatrienone is stable, but cyclopentadienone is so reactive that it can?t be isolated. Explain, taking the polarity of the carbonyl group into account. Cycloheptatrienone Cyclopentadienone
-
In the 2012 GSS, 605 of 790 males and 822 of 977 females indicated a belief in life after death. (Source: Data from CSM, UC Berkeley.) a. Construct a 2 2 contingency table relating gender of...
-
List and evaluate the significance of the risk list as a database of risks affecting organisations.
-
Elaborate in 500 words on the advantages that administrative officers and secretaries can gain by pursuing a degree in library and information science. Additionally, discuss the significance of...
-
Identify 5 key questions that HR leaders should consider specific to the trend of Redefining Remote and Hybrid Work Strategies.
-
Explain why including direction, control, and coordination are critical in writing your EOP? How do you ensure everyone is collaborating and performing the objectives outlined in the plan? Why or why...
-
1. Why is quality systems (such as Six Sigma) important for careers in engineering technology/engineering technology management? 2. Describe an instance when you have utilized production planning and...
-
Let \(\left\{X_{n}ight\}\) be a sequence of independent and identically distributed random variables where the distribution function of \(X_{n}\) is \[F_{n}(x)= \begin{cases}1-x^{-\theta} & \text {...
-
Will the prediction interval always be wider than the estimation interval for the same value of the independent variable? Briefly explain.
-
Nitrogen monoxide, a pollutant in automobile exhaust, is oxidized to nitrogen dioxide in the atmosphere according to the equation: Find K c for this reaction. 2 NO(g) + 0(8) 2 NO(8) Kp 2.2 x 102 at...
-
Suggest a method for the synthesis of the unnatural D enantiomer of alanine from the readily available L enantiomer of lactic acid. CH,-CHOH-COOH lactic acid
-
Show how you would use the Gabriel-malonic ester synthesis to make histidine. What stereochemistry would you expect in your synthetic product?
-
Show how you would use the Strecker synthesis to make tryptophan. What stereochemistry would you expect in your synthetic product?
-
During the fall season, a retailer determined that in order to meet the next season's planned sales, the total amount of merchandise required next season was $360,000 at retail, with an initial...
-
Explore the role of collateralized debt obligations (CDOs) and collateralized loan obligations (CLOs) in securitizing and tranched collateralized credit exposures. How do structured finance vehicles,...
-
The faculty members from a local school voted to determine the winner of the "Student of the Year" award. The candidates were Ivanna, Tony, and Michael. The voting ballots are summarized in the...

Study smarter with the SolutionInn App


