Danson Company is a chemical manufacturer that supplies various products to industrial users. The company plans to
Question:
Danson Company is a chemical manufacturer that supplies various products to industrial users. The company plans to introduce a new chemical solution, called Nysap, for which it needs to develop a standard product cost. The following information is available on the production of Nysap:
(a) Nysap is made by combining a chemical compound (nyclyn) and a solution (salex), and boiling the mixture. A 20% loss in volume occurs for both the salex and the nyclyn during boiling. After boiling, the mixture consists of 9.6 liters of salex and 12 kilograms of nyclyn per 10-liter batch of Nysap.
(b) After the boiling process is complete, the solution is cooled slightly before 5 kilograms of protect are added per 10-liter batch of Nysap. The addition of the protect does not affect the total liquid volume. The resulting solution is then bottled in 10-liter containers.
(c) The finished product is highly unstable, and one 10-liter batch out of five is rejected at final inspection. Rejected batches have no commercial value and are thrown out.
(d) It takes a worker 35 minutes to process one 10-liter batch of Nysap. Employees work eight hour day, including one hour per day for rest breaks and cleanup.
Required:
1. Determine the standard quantity for each of the raw materials needed to produce an acceptable 10-liter batch of Nysap.
2. Determine the standard labor time allowed to produce an acceptable 10-liter batch of Nysap.
3. Assuming the following costs, prepare a standard cost card for direct materials and direct labor for one acceptable 10-liter batch of Nysap:
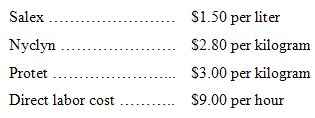
Step by Step Answer:

Managerial Accounting
ISBN: 978-0697789938
13th Edition
Authors: Ray H. Garrison, Eric W. Noreen, Peter C. Brewer





