Question: Draw the important resonance structures for these species and discuss the contribution of each to the resonance hybrid. Explain whether the species has a large
Draw the important resonance structures for these species and discuss the contribution of each to the resonance hybrid. Explain whether the species has a large or a small amount of resonance stabilization?
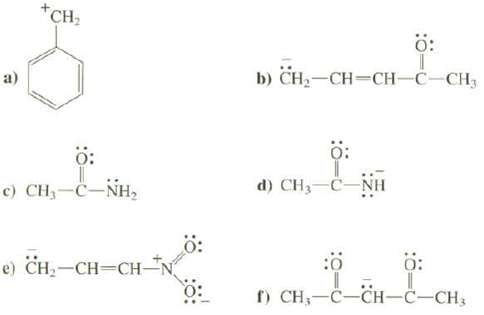
a) +CH : c) CH,CNH, e) CH-CH=CH- b) CH-CH=CH-C-CH3 : d) CH-C-NH : :0 : f) CH-C-CH-C-CH3
Step by Step Solution
3.49 Rating (186 Votes )
There are 3 Steps involved in it
a All of these resonance structures are important Although the carbocation is unstable because it do... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
15-C-O-C (118).docx
120 KBs Word File


