Question: For a given a hydrogen atom to be acidic, the C?H bond must be parallel to the p orbital?s of the C=O bond (that is,
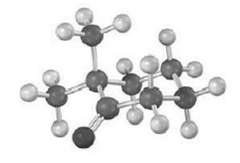
For a given a hydrogen atom to be acidic, the C?H bond must be parallel to the p orbital?s of the C=O bond (that is, perpendicular to the plane of the adjacent carbonyl group). Identify the most acidic hydrogen atom in the conformation shown for the following structure. Is it axial or equatorial?
Step by Step Solution
3.30 Rating (162 Votes )
There are 3 Steps involved in it
HC CH3 H Enolization can occur on only one side of the car... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
22-C-O-CA (188).docx
120 KBs Word File


