Question: From information in Table 15-3, explain how you would use KMnO 4 to find the content of (NH 4 ) 2 S 2 O 8
From information in Table 15-3, explain how you would use KMnO4 to find the content of (NH4)2S2O8 in a solid mixture with (NH4)2SO4. What is the purpose of phosphoric acid in the procedure?
Table 15-3
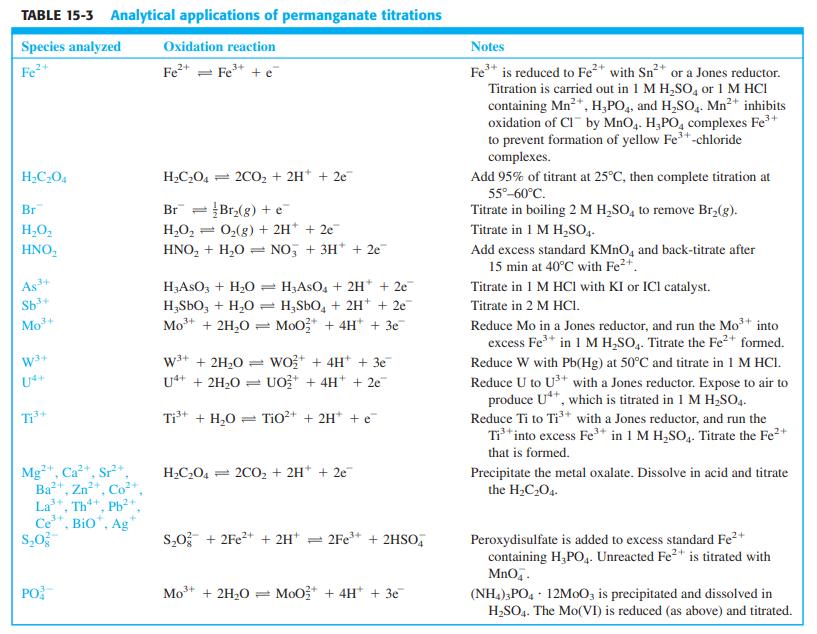
TABLE 15-3 Analytical applications of permanganate titrations Species analyzed Oxidation reaction Notes Fe+ Fe* = Fe* + e Fe* is reduced to Fe* with Sn* or a Jones reductor. Titration is carried out in 1 M H,SO, or 1 M HCI containing Mn2*, H,PO4, and H,SO,. Mn2+ inhibits oxidation of CI by MnO4. H,PO, complexes Fe* to prevent formation of yellow Fe+-chloride 2+ complexes. H;CO4 = 2CO, + 2H* + 2e Add 95% of titrant at 25C, then complete titration at 55-60C. Titrate in boiling 2 M H,SO, to remove Br,(g). Br Br:(g) + e = 0,(g) + 2H + 2e Br H,O, H,O, Titrate in 1 M H,SO,. HNO, HNO, + H,0 = NO, + 3H* + 2e Add excess standard KMNO, and back-titrate after 15 min at 40C with Fe2+. As 34 Sb* HASO; + H20 : H3ASO, + 2H* + 2e H;SbO, + H,0 = H,SbO, + 2H* + 2e MoO* + 4H* + 3e Titrate in 1 M HCI with KI or ICl catalyst. Titrate in 2 M HCI. Mo+ Mo + 2H,0 Reduce Mo in a Jones reductor, and run the Mot into excess Fe* in 1 M H,SO,. Titrate the Fe2+ formed. w+ + 2H20 wo* + 4H* + 3e UO* + 4H+ + 2e Reduce W with Pb(Hg) at 50C and titrate in 1 M HCI. U4+ Ut+ + 2H20 Reduce U to U*+ with a Jones reductor. Expose to air to produce U**, which is titrated in 1 M H;SO. Reduce Ti to Ti* with a Jones reductor, and run the Ti*into excess Fe+ in 1 M H,SO,. Titrate the Fe2+ +1 T Ti* + H,0 = Tio?+ + 2H* + e that is formed. Mg", Ca, Sr*. H2C204 = 2CO, + 2H* + 2e Precipitate the metal oxalate. Dissolve in acid and titrate the H2C2O4. Ba+ La", Th, Ph+ Ce", Bio", Ag Zn Co s,0 + 2Fe?+ + 2H* = 2Fe** + 2HSO, Peroxydisulfate is added to excess standard Fe2+ containing H,PO,. Unreacted Fe2" is titrated with MnO.. PO Mo+ + 2H20 = MoO* + 4H* + 3e (NH,);PO4 - 12M0O3 is precipitated and dissolved in H,SO4. The Mo(VI) is reduced (as above) and titrated.
Step by Step Solution
3.40 Rating (166 Votes )
There are 3 Steps involved in it
A weighed amount of the solid mixture is added to a solution containing ex... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
878-E-C-E-E-C (2081).docx
120 KBs Word File


