Question: In 1932, A. A. Levine and A. G. Cole studied the ozonolysis of o-xylene and isolated three products: glyoxal, 2, 3-butanedione, and pyruvaldehyde: In what
In 1932, A. A. Levine and A. G. Cole studied the ozonolysis of o-xylene and isolated three products: glyoxal, 2, 3-butanedione, and pyruvaldehyde: In what ratio would you expect the three products to be formed if o-xylene is a resonance hybrid of two structures? The actual ratio found was 3 parts glyoxal, 1 part 2, 3-butanedione, and 2 parts pyruvaldehyde. What conclusions can you draw about the structure ofo-xylene?
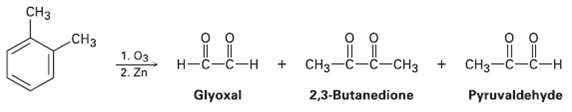
C CH 1. 2. Zn CH - CH3 Glyoxal 2,3-Butanedione Pyruvaldehyde
Step by Step Solution
3.37 Rating (181 Votes )
There are 3 Steps involved in it
CH3 A CH3 B CH3 CH3 103 2 Zn H3O 103 2 Zn HO I CO OC H CH3 C0 OC 0 H Mozo H O OC 00 CH... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
22-C-O-B (103).docx
120 KBs Word File


