Question: In absorption refrigerators the energy driving the process is supplied not as work, but as heat from a gas flame at a temperature hh
In absorption refrigerators the energy driving the process is supplied not as work, but as heat from a gas flame at a temperature τhh > τh. Mobile home and cabin refrigerators may be of this type, with propane fuel.
(a) Give an energy-entropy flow diagram similar to figures for such a refrigerator, involving no work at all, but with energy and entropy flows at the three temperatures τhh > τh > τl.
(b) Calculate the ratio Ql/Qhh, for the heat extracted at τ = τl, where Qhh is the heat input at τ = τhh. Assume reversible operation.
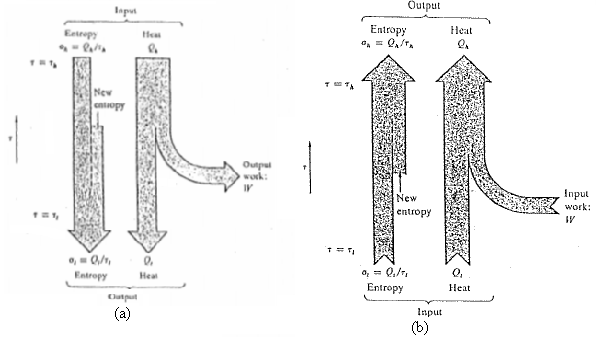
Output Ieput Entropy Heat Estrepy New entopy Outpul work: IV Input New work: entropy Entropy Hea Entropy Heat Clatput (a) Input (b)
Step by Step Solution
3.27 Rating (159 Votes )
There are 3 Steps involved in it
a See figure above which applies to this problems as well b From energy ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
42-P-S-S-T-T (82).docx
120 KBs Word File


