In crystals of the salt cesium chloride, cesium ions Cs+ form the eight corners of a cube
Question:
In crystals of the salt cesium chloride, cesium ions Cs+ form the eight corners of a cube and a chlorine ion Cl?? is at the cube's center (Figure). The edge length of the cube is 0.40 nm. The Cs+ ions are each deficient by one electron (and thus each has a charge of + e), and the Cl ?? ion has one excess electron (and thus has a charge of ?? e).(a) What is the magnitude of the net electrostatic force exerted on the Cl ?? ion by the eight Cs+ ions at the corners of the cube?(b) If one of the Cs+ ions is missing, the crystal is said to have a defect; what is the magnitude of the net electrostatic force exerted on the Cl?? ion by the seven remaining Cs+ions?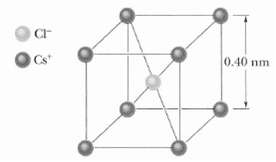
Step by Step Answer:

Fundamentals of Physics
ISBN: 978-0471758013
8th Extended edition
Authors: Jearl Walker, Halliday Resnick





