Question: Lead experiences corrosion in an acid solution according to the reaction The rates of both oxidation and reduction half-reactions are controlled by activation polarization. (a)
Lead experiences corrosion in an acid solution according to the reaction
The rates of both oxidation and reduction half-reactions are controlled by activation polarization.
(a) Compute the rate of oxidation of Pb (in mol/cm2-s) given the following activation polarization data:
(b) Compute the value of the corrosionpotential.
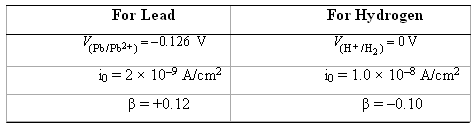
For Lead P/P62+) =-0.126 V For Hydrogen '(H+ /H2) = OV i0 = 2 x 10-9 A/cm? B= +0.12 i0 = 1.0 x 10-8 A/cm2 B=-0.10
Step by Step Solution
3.35 Rating (182 Votes )
There are 3 Steps involved in it
a This portion of the problem asks that we compute the rate of oxidation for Pb given that both the ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
33-E-M-S-E-M-S (660).docx
120 KBs Word File


