Liquid air is fed to the top of a perforated-tray reboiled stripper operated at substantially atmospheric pressure.
Question:
Liquid air is fed to the top of a perforated-tray reboiled stripper operated at substantially atmospheric pressure. Sixty percent of the oxygen in the feed is to be drawn off in the bottoms vapor product from the still. This product is to contain 0.2 mol% nitrogen. Based on the assumptions and data given below, calculate:
(a) The mole percent of nitrogen in the vapor leaving the top plate.
(b) The moles of vapor generated in the still per 100 mol of feed.
(c) The number of theoretical platesrequired.
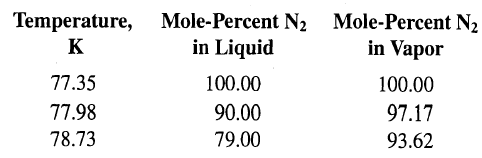

Transcribed Image Text:
Temperature, Mole-Percent N2 Mole-Percent N2 in Vapor in Liquid K 77.35 77.98 78.73 100.00 100.00 90.00 97.17 79.00 93.62
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (12 reviews)
Subject Separation of air in a reboiled stripper Given Reboiled stripper with total reboiler operati...View the full answer

Answered By

Umber Talat
I am providing full time mentoring and tutoring services in Business Finance, Contemporary issue in Global Economy, Quantitative Techniques, Principles of Marketing, strategic marketing, International Marketing, Organizational Behavior (OB), Consumer Behavior, Sales Force Management, Strategic Brand Management, Services Marketing, Integrated Marketing Communication (IMC), Principles of Management, General Management, Strategic Management, Small and Medium Enterprise Management, Innovation Management, Change Management, Knowledge Management, Strategic Planning, Operations Management, Supply Chain Management, Logistics Management, Inventory management, Total Quality Management (TQM), Productions Management, Project Management, Production Planning, Human Resource Management (HRM), Human Resource Development, Strategic HRM, Organizational Planning, Performance and Compensation Management, Recruitment and Selection, Organizational Development, Global Issues in Human Resource Management, Retail Marketing, Entrepreneurship, Entrepreneurial Marketing, International Business, Research Methods in Business, Business Communication, Business Ethics.
4.70+
158+ Reviews
236+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Chemical Engineering questions
-
A firehouse must be able to shoot water to the top of a building 35.0 m tall when aimed straight up. Water enters this hose at a steady rate of 0.500 m'/s and shoots out of a round nozzle. (a) What...
-
A ski gondola is connected to the top of a hill by a steel cable of length 620 m and diameter 1.5cm. As the gondola comes to the end of its run, it bumps into the terminal and sends a wave pulse...
-
A 65-kg hiker climbs to the top of a 3700-m-high mountain. The climb is made in 5.0h starting at an elevation of 2300m. Calculate (a) The work done by the hiker against gravity, (b) The average power...
-
Why would senior managers want to distort factor prices by using cost allocations?
-
The financial statements of Eastern Platinum Limited (Eastplats) are presented in Appendix A at the end of this book. Instructions (a) Some companies list not only accounts payable but accrued...
-
A force P is applied to a bent rod ABC, which may be supported in four different ways as shown. In each case, if possible, determine the reactions at the supports. 450 -300 el 30 d)
-
Find the probability of being dealt 5 diamonds from a standard deck of 52 playing cards.
-
Multiple Choice Questions 1. The Hickory Company made a lump-sum purchase of three pieces of machinery for $115,000 from an unaffiliated company. At the time of acquisition Hickory paid $5,000 to...
-
Chatman has pled guilty to possession of controlled substances and is pending sentencing. His attorney has told him that the judge usually orders everyone drug tested at sentencing and the result...
-
A physics student pulls a block of mass m = 22 kg up an incline at a slow constant velocity for a distance of d = 4.5 m. Does the incline make an angle? = 32? with the horizontal. The coefficient of...
-
(a) For the cascade shown in Figure a, calculate the compositions of streams V4 and L1. Assume atmospheric pressure, saturated liquid and vapor feeds, and the vapor-liquid equilibrium data given...
-
A mixture of A (more volatile) and B is being separated in a plate distillation column. In two separate tests run with a saturate- liquid feed of 40 mol% A, the following compositions, in mol% A were...
-
Follow the steps described in the chapter to manage your online reputation. What did you find in a thorough search that included different search engines, name and term combinations, and videos and...
-
If you are part of a remote team, develop a charter to agree on how youll work together. Refer to Figure 3 for questions about your purpose, results, communication, roles and responsibilities,...
-
Find and compare diversity, inclusion, and/or belonging statements from two or three of your favorite companies. Answer the following questions, and then share your responses with another student: ...
-
In small groups, discuss text messages you received recently that caused misunderstandings. What was the situation and what went wrong? If you can, share the messages to gauge how your classmates...
-
Use your voice tone to convey emotions. With a partner, practice conveying different emotions. If you go first, read the sentences in the left-side column, changing your tone to reflect each emotion....
-
Imagine that you work for a wealth management firm, which manages financial assets for individuals. You have a prospective new client, whose previous financial manager was her brother-in-law, who...
-
In what circumstances might a director have a conflict of interest?
-
Factor and simplify, if possible. Check your result using a graphing calculator. 3 cot 2 + 6 cot + 3
-
Distinguish between the general principles and the standards of the APA ethics code. Describe any three of the general principles, as they apply to research.
-
What advantages do the MaxwellStefan relations provide for multicomponent mixtures containing charged biomolecules, in comparison with Ficks law?
-
What is the two-film theory of Whitman? Is equilibrium assumed to exist at the interface of two phases?
-
For mass transfer across a phase interface, what is the difference between the film, penetration, and surface-renewal theories, particularly with respect to the dependence on diffusivity?
-
A corporation issues 13 %, 15-year bonds with a par value of $570,000 and semiannual interest payments. On the issue date, the annual market rate for these bonds is 11%, which implies a selling price...
-
If you were asked whether a large university such as Tennessee or Michigan with a large seating capacity for their football stadiums should build a new football stadium, how would you respond and...
-
A production department reports the following conversion costs. Equivalent units of production for conversion total 436,000 units this period. Calculate the cost per equivalent unit of production for...

Study smarter with the SolutionInn App


