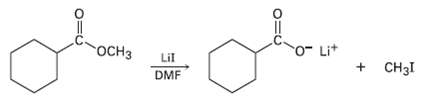
Methyl esters (RCO2CH3) undergo a cleavage reaction to yield carboxylate ions plus iodomethane on heating with LiI
Question:
Methyl esters (RCO2CH3) undergo a cleavage reaction to yield carboxylate ions plus iodomethane on heating with LiI in dimethyl form amide: The following evidence has been obtained: (1) the reaction occurs much faster in DMF than in ethanol. (2) The corresponding ethyl ester (RCO2CH2CH3) cleaves approximately 10 times more slowly than the methyl ester. Propose a mechanism for the reaction. What other kinds of experimental evidence could you gather to support yourhypothesis?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





