Question: Refer to the drawing that accompanies Problem 13. When a system changes from A to B along the path shown on the pressure-versus-volume graph, it
Refer to the drawing that accompanies Problem 13. When a system changes from A to B along the path shown on the pressure-versus-volume graph, it gains 2700 J of heat. What is the change in the internal energy of the system?
Data from Problem 13:
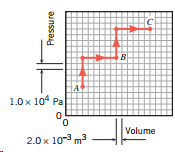
1.0x 104 Pa Volume 2.0 x 10-3 m3 Pressure
Step by Step Solution
3.48 Rating (164 Votes )
There are 3 Steps involved in it
According to the first law of thermodynamics the change in internal e... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
1176-E-M-E-T(8070).docx
120 KBs Word File


