Saturated steam at a gauge pressure of 2.0bar is to be used to heat a stream of
Question:
Saturated steam at a gauge pressure of 2.0bar is to be used to heat a stream of ethane. The ethane enters a heat exchanger at 16?C and 1.5 bar gauge at a rate of 795m3/min and is heated at constant pressure to 93?C. The steam condenses and leaves the exchanger as a liquid at 27?C. The specific enthalpy of ethane at the given pressure is 941kJ/kg at 16?C and 1073 kJ/kg at 93?C.
(a) How much energy (kW) must be transferred to the ethane to heat it from 16?C to 93?C?
(b) Assuming that all the energy transferred from the steam goes to heat the ethane, at what rate in m3/s must steam be supplied to the exchanger? If the assumption is incorrect, would the calculated value be too high or too low?
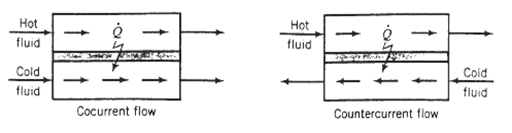
(c) Should the heat exchanger be set up for concurrent or countercurrent flow (see the following schematic diagram)? Explain.?
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-0471720638
3rd Edition
Authors: Richard M. Felder, Ronald W. Rousseau





