Question: Some consequences of the Maxwell-Boltzmann distribution, in the simplified kinetic theory in S1.4, several statements concerning the equilibrium behavior of a gas were made without
Some consequences of the Maxwell-Boltzmann distribution, in the simplified kinetic theory in S1.4, several statements concerning the equilibrium behavior of a gas were made without proof. In this problem and the next, some of these statements are shown to be exact consequences of the Maxwell-Boltzmann velocity distribution. The Maxwell-Boltzmann distribution of molecular velocities in an ideal gas at rest is f(ux, uy, uz) = n(m/2?kT)3/2 exp(?mu2/2kT) (1C.1-1) in which u is the molecular velocity, n is the number density, and f(ux, uy, uz)dux?duy?duz is the number of molecules per unit volume that is expected to have velocities between ux and ux + dux, uy and uy + duy, uz and uz + duz. It follows from this equation that the distribution of the molecular speed u is f(u) = 4?nu2(m/2?kT)3/2 exp(?mu2 /2kT)?
(a) Verify Eq. 1.4-1 by obtaining the expression for the mean speed u from
(b) Obtain the mean values of the velocity components ux, uy, and uz. The first of these is obtained from what can one conclude from the results?
(c) Obtain the mean kinetic energy per molecule by the correct result is ? mu2 = 3/2kT.
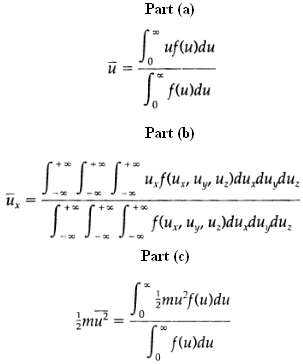
Ux Part (a) Suf(u)du 0 fflurdu Part (b) u.f(u, u, u.)du du du. ** ** ** f(u, u, udududu. Part (c) mu S* \muf(u)du S flurdu
Step by Step Solution
3.38 Rating (164 Votes )
There are 3 Steps involved in it
z Some consequences of the MaxwellBoltzmann equation a The mean spee... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
6-E-C-E-T-P (11).docx
120 KBs Word File


