Question: Sometimes it is desirable to be able to determine the weight percent of one element, C 1 that will produce a specified concentration in terms
Sometimes it is desirable to be able to determine the weight percent of one element, C1 that will produce a specified concentration in terms of the number of atoms per cubic centimeter, N1, for an alloy composed of two types of atoms. This computation is possible using the following expression:

Where
NA = Avogadro’s number
ρ1 and ρ2 = densities of the two elements
A1 and A2 = the atomic weights of the two elements
Derive Equation 4.19 using Equation 4.2 and expressions contained in Section 4.4.
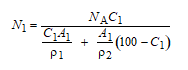
100 C = (4.19) NP2 P2 1+ N,4
Step by Step Solution
3.36 Rating (162 Votes )
There are 3 Steps involved in it
The number of atoms of component 1 per cubic centime... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
33-E-M-S-E-M-S (112).docx
120 KBs Word File


