Question: The specific heat at constant pressure cp [J/(kg K)] of an ideal gas is related to enthalpy by where h = enthalpy (kJ/kg), and
The specific heat at constant pressure cp [J/(kg · K)] of an ideal gas is related to enthalpy by
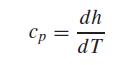
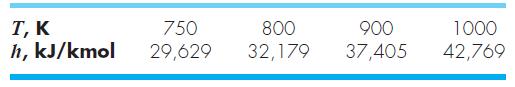
where h = enthalpy (kJ/kg), and T = absolute temperature (K). The following enthalpies are provided for carbon dioxide (CO2) at several temperatures. Use these values to determine the specific heat in J/(kg · K) for each of the tabulated temperatures. Note that the atomic weights of carbon and oxygen are 12.011 and 15.9994 g/mol, respectively
Cp = dh dT
Step by Step Solution
3.46 Rating (159 Votes )
There are 3 Steps involved in it
To determine the specific heat at constant pressure cp in Jkg K for each of the tabulated temperatur... View full answer

Get step-by-step solutions from verified subject matter experts


