Question: Below, I have shown a true statement about several pairs of molecules. You need to assign the appropriate rationale for each true statement in
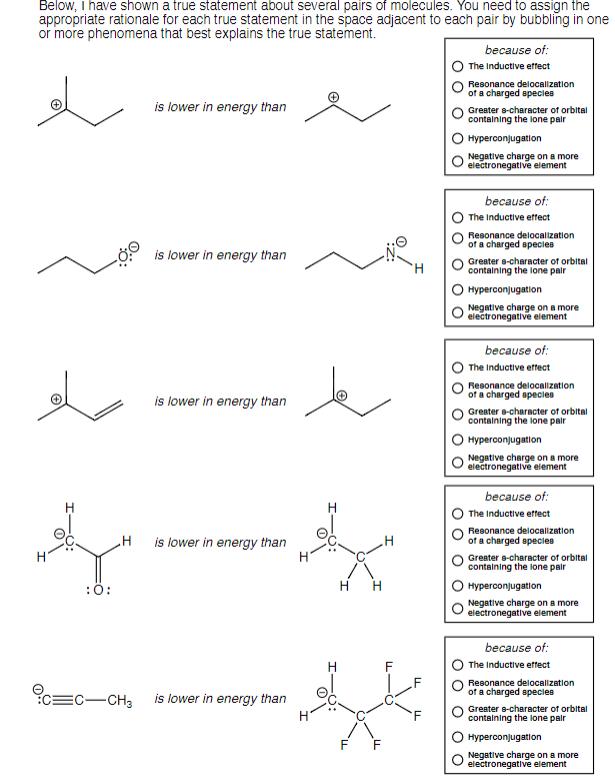
Below, I have shown a true statement about several pairs of molecules. You need to assign the appropriate rationale for each true statement in the space adjacent to each pair by bubbling in one or more phenomena that best explains the true statement. :O: H EC-CH3 is lower in energy than is lower in energy than is lower in energy than is lower in energy than is lower in energy than H H H H F because of: The Inductive effect Resonance delocalization of a charged species Greater 8-character of orbital containing the lone pair Hyperconjugation Negative charge on a more electronegative element because of: The Inductive effect Resonance delocalization of a charged species Greater a-character of orbital containing the lone pair Hyperconjugation Negative charge on a more electronegative element because of: The Inductive effect Resonance delocalization of a charged species Greater a-character of orbital containing the lone pair Hyperconjugation Negative charge on a more electronegative element because of: The Inductive effect Resonance delocalization of a charged species Greater a-character of orbital containing the lone pair Hyperconjugation Negative charge on a more electronegative element because of: The Inductive effect Resonance delocalization of a charged species Greater e-character of orbital containing the lone pair Hyperconjugation Negative charge on a more electronegative element
Step by Step Solution
3.44 Rating (151 Votes )
There are 3 Steps involved in it
Pair 1 In this pair H2O is the first molecule while H2S is the second Compared to the sulfur atom in H2S the oxygen atom in H2O is more electronegative This implies that the hydrogen atoms will retain ... View full answer

Get step-by-step solutions from verified subject matter experts


