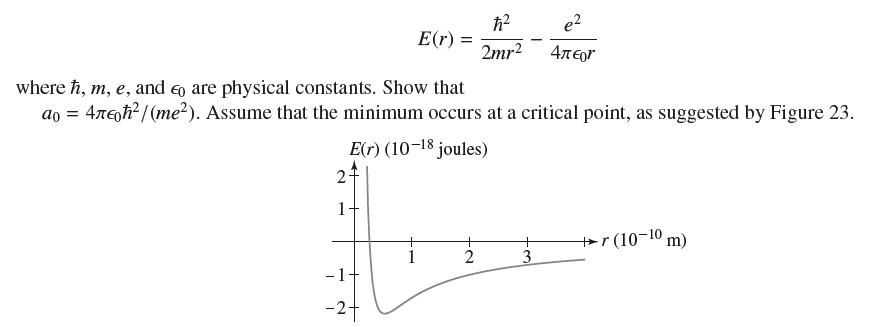
The Bohr radius a 0 of the hydrogen atom is the value of r that minimizes the
Question:
The Bohr radius a0 of the hydrogen atom is the value of r that minimizes the energy
Transcribed Image Text:
2 1- E(r) = where ħ, m, e, and o are physical constants. Show that ao = 4лεħ²/(me²). Assume that the minimum occurs at a critical point, as suggested by Figure 23. E(r) (10-18 joules) - 1 -24 ħ² 2mr² 2 + e² 4πeor 3 +r (10-10 m)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
Let Then impli...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Mathematics questions
-
The hydrogen atom is composed of one proton in the nucleus and one electron, which moves about the nucleus. In the quantum theory of atomic structure, it is assumed that the electron does not move in...
-
The ground-state wave function of a hydrogen atom is = (1/xa3)1/2e-r1ao where ao = 53 pm (the Bohr radius). (a) Calculate the probability that the electron will be found somewhere within a small...
-
The wave function for the 2pz orbital in the hydrogen atom is Where a0 is the value for the radius of the first Bohr orbit in meters (5.29 Ã 10-11), Ï is Zr/a0, r is the value for the...
-
Two wires carrying equal and opposite currents are twisted together in the construction of a circuit. Why does this technique reduce stray magnetic fields? Please explain for dummies.
-
A corporation issued $1,000,000 of common stock in exchange for $1,000,000 of fixed assets. Where would this transaction be reported on the statement of cash flows?
-
A four-bladed helicopter rotor rotates at n r/min in air with properties (ρ, μ). Each blade has chord length C and extends from the center of rotation out to radius R (the hub size is...
-
Determine if the mixed method aspect is concurrent, sequential,, or nested. Develop a hypothetical research scenario that would necessitate the use of the Mixed Method A-B-A Design. The research will...
-
Davis Industries must choose between a gas-powered and an electric-powered forklift truck for moving materials in its factory. Since both forklifts perform the same function, the firm will choose...
-
A 3.0-cm tall object is 55 cm in front of a diverging mirror that has a -20 cm focal length. Part A Calculate the image position. Input a positive value if the image is on the same side from the...
-
What is the potential of a copper electrode immersed in 0.0380 M in Cu (NO3)2 0.0650 M in NaCl and saturated with CuCl 0.0350 M in NaOH qnd saturated with Cu(OH)2 0.0375 M in Cu (NH3)4^2+ and 0.108 M...
-
The response of a circuit or other oscillatory system to an input of frequency (omega) is described by the function 50 () = @ (A) D = 0.01 Both wo (the natural frequency of the system) and D (the...
-
Prove that x = 4 is the greatest root of (x) = x 4 8x 2 128.
-
Meng Group is preparing the comparative financial statements for the annual report to its shareholders for fiscal years ended May 31, 2019, and May 31, 2020 (yen in thousands). The income from...
-
Compute Ms. Perez's preparer penalty if the IRS concludes that Ms. Perez intentionally disregarded the tax law by claiming the deduction in a willful attempt to understate Denver's tax. Compute Ms....
-
Now that you know what you want to present to an audience, it is important to fashion it into an active, engaging oral presentation. How you look, express yourself, and engage with your audience may...
-
Company JBY is asking your help in providing the management an idea how much is the Company's the cost of capital. After your initial meeting, you recommended to use Weighted Average Cost of Capital...
-
RANDLEMAN, N.C. -- The future of century-old bearing maker Timken Co. rests on an old axiom: The sum is greater than its parts. Examples fill the shelves of a squat, 10-year-old factory here crammed...
-
Question content area bottom Part 1 Included in Post-Closing Trial Balance? a. Office Supplies Y b. Interest Expense N c. Retained Earnings d. Dividends Y e. Service Revenue N f. Accumulated...
-
The management of The Hershey Company has asked union workers in two of its highest cost Pennsylvania plants to accept higher health insurance premiums and take a wage cut. The workers portion of the...
-
Study the pictures/images below. Obviously these was focus on LT sociology, anthropology and poltical science. Try to do some analysis by finding clues that are synonymous with the main concepts....
-
Which function approaches 0 faster as x approaches infinity: e-x or 1/x? Many improper integrals can be evaluated by comparing functions with the method of leading behavior. State which of the given...
-
Evaluate the following improper integrals or say why they don't converge. dx 0 (2+5x
-
Use the comparison test to deduce whether the following improper integrals converge. If they do, find an upper bound on the value. dx
-
A campus network is connected to the Internet via an access link with 100 Mb/s upload rate and practically unlimited download rate. Sixty percent of the access link bandwidth is used to access the...
-
[2] [2] [2] [2] [2] 1. Short answer questions. Provide brief justification for your answers. (a) In how many ways can 51 red marbles and 37 blue marbles be distributed to 13 children if each child is...
-
6) Company A has been allocated the Class B address block 177.248.0.0/16. Internally, they want to break this address block into 32 subnets of equal size. a.) What Subnet Mask should be used on...

Study smarter with the SolutionInn App


