Iron is extracted from its ores haematite and magnetite. Usually it is extracted from haematite (iron(iii) oxide).
Question:
Iron is extracted from its ores haematite and magnetite. Usually it is extracted from haematite (iron(iii) oxide). The ore is mixed with limestone and coke and reduced to the metal in a blast furnace. The following is a brief outline of the reactions involved.
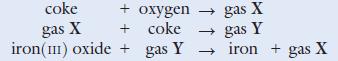
a. Name the gases X and Y.
b. Give a chemical test to identify gas X.
c. Write balanced chemical equations for the reactions shown above.
d. The added limestone is involved in the following reactions:
limestone → calcium oxide + gas X
calcium oxide + silicon(iv) oxide → slag
(i) Give the chemical names for limestone and slag.
(ii) Write balanced chemical equations for the reactions shown above.
(iii) Why is the reaction between calcium oxide and silicon(iv) oxide called an acid–base reaction?
(iv) Describe what happens to the liquid iron and slag when they reach the bottom of the furnace.
e. Why do you think that the furnace used in the extraction of iron is called a blast furnace?
Step by Step Answer:






