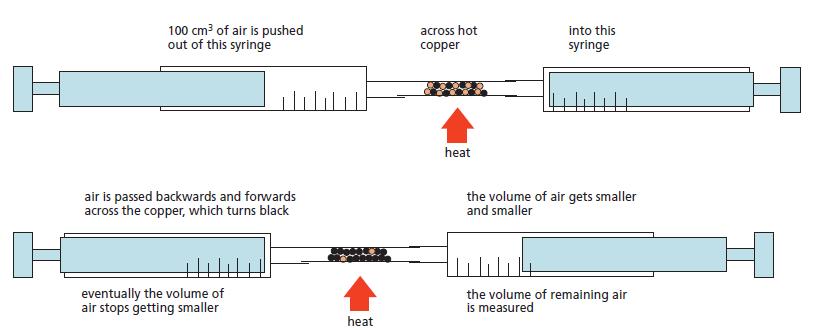
The apparatus shown on Figure 11.6, was used to estimate the proportion of oxygen in the atmosphere.
Question:
The apparatus shown on Figure 11.6, was used to estimate the proportion of oxygen in the atmosphere. A volume of dry air (200 cm3) was passed backwards and forwards over heated copper until no further change in volume took place. The apparatus was then allowed to cool down to room temperature and the final volume reading was then taken. Some typical results are shown below.
Volume of gas before = 200 cm3
Volume of gas after = 157 cm3
During the experiment the copper slowly turned black.
a. Why was the apparatus allowed to cool back to room temperature before the final volume reading was taken?
b. Using the information given above, calculate the percentage volume reduction which has taken place.
c. Explain briefly why there is a change in volume.
d. What observation given above supports your explanation in c? Write a balanced chemical equation for any reaction which has occurred.
e. Give the name of the main residual gas at the end of the experiment.
f. Would you expect the copper to have increased or decreased in mass during the experiment? Explain your answer.
Figure 11.6
Step by Step Answer:






