Question: 14. Table 5.5 shows the potential differences across the electrodes of electrochemical cells made using different combinations of metals. Metals used to make the cell
14. Table 5.5 shows the potential differences across the electrodes of electrochemical cells made using different combinations of metals.
Metals used to make the cell
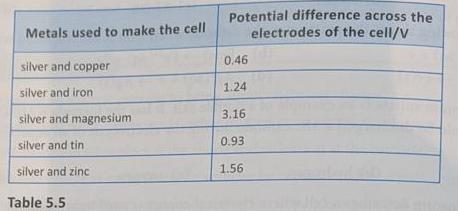
(a) The greater the difference in reactivity of the metals the greater the potential difference between them. Which of the metals in Table 5.5 is:
(i) nearest in reactivity to silver
(ii) farthest in reactivity from silver?
(b) Use the data in the table to draw a reactivity series for the six metals mentioned, taking silver as the least reactive of them.
(c) Use the data in Table 5.5 and predict the potential difference between the following metals when in an electrochemical cell.
(i) magnesium and conner
Metals used to make the cell Potential difference across the electrodes of the cell/V silver and copper 0.46 silver and iron 1.24 silver and magnesium 3.16 silver and tin 0.93 silver and zinc 1.56 Table 5.5
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


