1-bromobutane will undergo reactions when heated, as shown by reactions A and B. a. For reactions A...
Question:
1-bromobutane will undergo reactions when heated, as shown by reactions A and B.
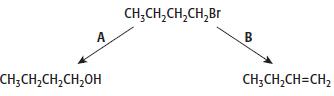
a. For reactions A and B give the reagents used in each case.
b. Reaction A was repeated using 1-iodobutane instead of 1-bromobutane. Explain any difference in the rate of reaction observed.
c. What type of organic reaction is A?
d. Show the mechanism for reaction A.
e. Reaction A was repeated with 2-bromo-2-methylpropane instead of 1-bromobutane.
i. Name the organic compound formed.
ii. The mechanism of the reaction with 2-bromo-2-methylpropane differs from the mechanism of reaction A. Describe how the mechanisms differ.
f. What type of reaction is B?
g. If reaction B was repeated with 2-bromobutane, name the other organic products that can form as well as the product shown above.
Step by Step Answer:

Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris





