a i. Explain whether the reaction below is an example of heterogeneous or homogeneous catalysis: ii. Explain
Question:
a i. Explain whether the reaction below is an example of heterogeneous or homogeneous catalysis:
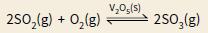 ii. Explain how a catalyst increases the rate of reaction in terms of activation energy and the distribution of molecular energies in a sample of reactants.
ii. Explain how a catalyst increases the rate of reaction in terms of activation energy and the distribution of molecular energies in a sample of reactants.
b. Enzymes are used in the biotechnology industry. They are far more efficient than traditional catalysts used in industrial processes. What would be the benefits of using enzymes instead of traditional catalysts in the industrial production of a chemical?
c. Draw an energy profile diagram to show a typical uncatalysed reaction and an enzyme-catalysed reaction. On your diagram show:
i. The activation energy for the catalysed and uncatalysed reactions
ii. The enzyme, substrate and product and enzyme–substrate intermediate.
Step by Step Answer:

Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris





