a State the electronic configurations of the following atoms or ions: i. Ti ii. Cr iii. Co
Question:
a State the electronic configurations of the following atoms or ions:
i. Ti
ii. Cr
iii. Co
iv. Fe3+
v. Ni2+
vi. Cu+
b. Explain why scandium (which forms only one ion, Sc3+) and zinc (which forms only one ion, Zn2+) are not called transition elements.
c. Why is the maximum oxidation state of manganese +7?
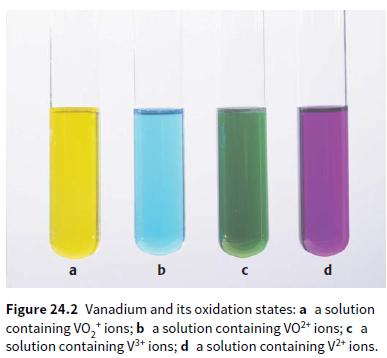
d. Look back at the different oxidation states of vanadium shown in Figure 24.2. State the oxidation state of the vanadium in each photo a–d.
e. Zirconium (Zr) is in the second row of transition elements beneath titanium in the Periodic Table. Its electronic configuration is [Kr]4d2 5s2, where [Kr] represents the electronic configuration of krypton, the noble gas with atomic number 36.
i. Predict the maximum stable oxidation state of zirconium and explain your answer.
ii. Give the formula of the oxide of zirconium, assuming zirconium exhibits the oxidation state
given in e, part i.
Step by Step Answer:

Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris





