a. Write a balanced chemical equation, including state symbols, for the reaction of: i. Strontium with oxygen
Question:
a. Write a balanced chemical equation, including state symbols, for the reaction of:
i. Strontium with oxygen
ii. Strontium oxide with water.
b. i. Write a balanced chemical equation, including state symbols, for the reaction of barium with water.
ii. Predict the pH of the solution formed in part b i.
c. Radium (Ra) is a radioactive element found below barium at the bottom of Group 2. Predict:
i. The formula of its ion
ii. The formula of its oxide and hydroxide
iii. Its first ionisation energy
iv. Its reactivity compared with barium
v. The relative pH of its saturated hydroxide solution compared with a saturated solution of calcium hydroxide
vi. The solubility of its sulfate compared with strontium sulfate.
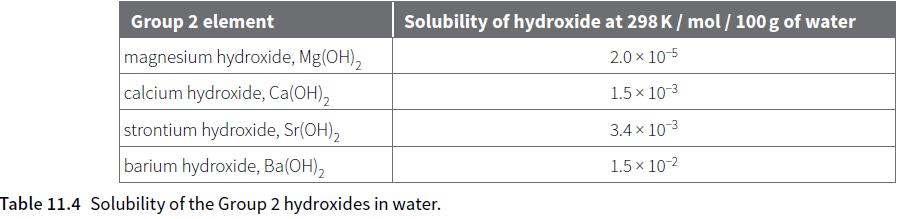
d. Using Table 11.4 and the relative atomic mass data, calculate the mass of calcium hydroxide that will dissolve in 50 g of water at 298 K.
The Periodic Table of the Elements
Step by Step Answer:

Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris





