a. Make the best estimate you can of the composition of the vapor in equilibrium with a
Question:
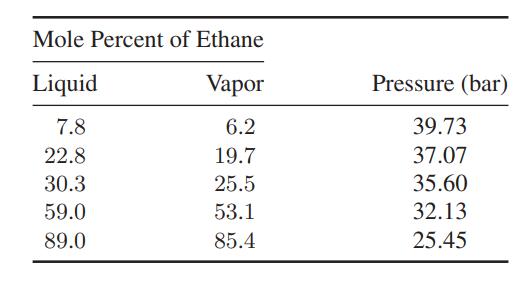
a. Make the best estimate you can of the composition of the vapor in equilibrium with a liquid containing 30.3 mol % ethane and 69.7 mol % ethylene at −0.01°C. Compare your results with the experimental data in the table.
b. Repeat the calculation in part (a) at other compositions for which the experimental data below are available.
Transcribed Image Text:
Mole Percent of Ethane Liquid Vapor 7.8 6.2 22.8 19.7 30.3 25.5 59.0 53.1 89.0 85.4 Pressure (bar) 39.73 37.07 35.60 32.13 25.45
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
a Calculate the composition of the vapor in equilibrium with a liquid containing 303 mol ethane and 697 mol ethylene at 001C To estimate the compositi...View the full answer

Answered By

Charan Mandhu
From my pre university course onwards i am teaching the students and online tutoring.I am about to complete my electronics in 1 months and i am good at computer science as well electrical iam capable to teach these subjects wherever student wants.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:
Students also viewed these Engineering questions
-
Redo Problem 10.3-7 using Aspen Plus. Problem 10.3-7 a. Make the best estimate you can of the composition of the vapor in equilibrium with a liquid containing 30.3 mol % ethane and 69.7 mol %...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Track Rack is a software development company that specializes in developing information systems to account for inventory. The company maintains an attractive website, which is one of its primary...
-
A trainee in a medical lab will be released to work on her own when her results agree with those of an experienced worker at the 95% confidence level. Results for a blood urea nitrogen analysis are...
-
A summary of revenues and expenses for Stanton Company for 2008 follows: Sales . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . $6,000,000 Cost of goods manufactured and sold ....
-
What are the defining characteristics of capitalism?
-
An electric motor, of mass \(20 \mathrm{~kg}\) and operating speed \(1350 \mathrm{rpm}\), is placed on a fixed-fixed steel beam of width \(15 \mathrm{~cm}\) and depth \(1.2 \mathrm{~cm}\), as shown...
-
1. How should Jennifer go about making her decision? 2. What kind of additional data or information should she collect? 3. What exactly should Jennifer require the others to submit in the way of...
-
1. Consider the following polynomial function graph of f(x) (use the letters as a guide) a) State the interval(s) over which f'(x) is positive. b) State the interval(s) over which f"(x) is negative....
-
Consider a stock currently trading at $100. A manager is considering to buy a 2-year at-the-money put option on the stock. For the valuation of this put option, the manager has selected binomial...
-
Vapor-liquid equilibria in petroleum technology are usually expressed in terms of K factors K i = y i /x i , where y i and x i are the mole fractions of species i in the vapor and liquid phases,...
-
a. Develop an algorithm for the equation-of-state prediction of the dew point pressure. b. Develop an algorithm for the equation-of-state prediction of the dew point temperature.
-
Slow Ride Corp. is evaluating a project with the following cash flows: Year Cash Flow 0....$29,000 1....11,200 2....13,900 3....15,800 4....12,900 5....-9,400 The company uses a 10 percent interest...
-
Badura Bombs, Inc's CEO, that evil genius known as "The Professor," is considering eliminating the unprofitable "Shark Head Laser" from their staple of products. The Shark Head Laser currently...
-
As at December 31, 2023, Bonhomme Inc. has the following shares outstanding: Common shares, 143,000 issued Preferred shares, $3.00 cumulative, 11,000 issued $1,143,000 $560,000 Dividends have not...
-
The following accounts pertain to Ali and Basil partnership: cash 10000, non-cash assets 90000, liabilities 30000, Ali capital 50000 and Basil capital 20000. The income ration 3:2. The partnership...
-
On May 20, Indy Landscapers received $3,000 cash in advance from one of its customers for lawn services it will provide in June. On June 4, Indy Landscapers provided the lawn services to its...
-
loan. On July 1, 2023, Ryan took out a new employee loan to purchase a bond which paid $3,000 interest income in the year. (Note: Ryan follows the cash method when recording interest income.) Ryan...
-
Consider the following. a. Calculate the leverage-adjusted duration gap of an FI that has assets of $ 1 million invested in 30-year, 10 percent semiannual coupon Treasury bonds selling at par and...
-
Distinguish among total-moisture content, free-moisture content, equilibrium-moisture content, unbound moisture, and bound moisture.
-
Find the pH of 0.050 M sodium butanoate (the sodium salt of butanoic acid, also called butyric acid).
-
The pH of 0.10 M ethylamine is 11.82. (a) Without referring to Appendix G, find Kb for ethylamine. (b) Using results from part (a), calculate the pH of 0.10 M ethylammonium chloride.
-
Which of the following bases would be most suitable for preparing a buffer of pH 9.00? (i) NH 3 (ammonia, K b = 1.76 10 -5 ); (ii) C 6 H 5 NH 2 (aniline, K b = 3.99 10 -10 ); (iii) H 2 NNH 2...
-
A 5.0 g bullet moving 325 m/s is shot into a 1.25 kg block, which slides for 1.35 seconds across the surface it is on before coming to rest. What is the average force of kinetic friction between the...
-
Some enterprising physics students working on a catapult decide to have a water balloon fight in the school hallway. The ceiling is of height 3 . 4 m, and the balloons are launched at a velocity of 9...
-
1-Define electric fields and how it helps us understand electricity. 2-Electric fields are represented as a physical effect of a configuration of charges that is created by the attraction of electric...

Study smarter with the SolutionInn App


